Hnrnpk protects against osteoarthritis through targeting WWC1 mRNA and inhibiting Hippo signaling pathway
- PMID: 38414246
- PMCID: PMC11081807
- DOI: 10.1016/j.ymthe.2024.02.027
Hnrnpk protects against osteoarthritis through targeting WWC1 mRNA and inhibiting Hippo signaling pathway
Abstract
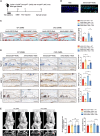
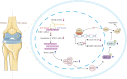
Osteoarthritis (OA) is an age-related or post-traumatic degenerative whole joint disease characterized by the rupture of articular cartilage homeostasis, the regulatory mechanisms of which remain elusive. This study identifies the essential role of heterogeneous nuclear ribonucleoprotein K (hnRNPK) in maintaining articular cartilage homeostasis. Hnrnpk expression is markedly downregulated in human and mice OA cartilage. The deletion of Hnrnpk effectively accelerates the development of post-traumatic and age-dependent OA in mice. Mechanistically, the KH1 and KH2 domain of Hnrnpk bind and degrade the mRNA of WWC1. Hnrnpk deletion increases WWC1 expression, which in turn leads to the activation of Hippo signaling and ultimately aggravates OA. In particular, intra-articular injection of LPA and adeno-associated virus serotype 5 expressing WWC1 RNA interference ameliorates cartilage degeneration induced by Hnrnpk deletion, and intra-articular injection of adeno-associated virus serotype 5 expressing Hnrnpk protects against OA. Collectively, this study reveals the critical roles of Hnrnpk in inhibiting OA development through WWC1-dependent downregulation of Hippo signaling in chondrocytes and defines a potential target for the prevention and treatment of OA.
Keywords: Hippo signaling; HnRNPK; WWC1; chondrocytes; osteoarthritis.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interest.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

