Activation of the YAP/KLF5 transcriptional cascade in renal tubular cells aggravates kidney injury
- PMID: 38414248
- PMCID: PMC11081877
- DOI: 10.1016/j.ymthe.2024.02.031
Activation of the YAP/KLF5 transcriptional cascade in renal tubular cells aggravates kidney injury
Abstract
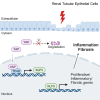
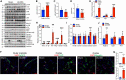
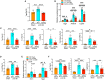
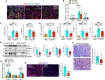
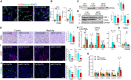
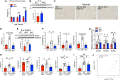
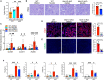
The Hippo/YAP pathway plays a critical role in tissue homeostasis. Our previous work demonstrated that renal tubular YAP activation induced by double knockout (dKO) of the upstream Hippo kinases Mst1 and Mst2 promotes tubular injury and renal inflammation under basal conditions. However, the importance of tubular YAP activation remains to be established in injured kidneys in which many other injurious pathways are simultaneously activated. Here, we show that tubular YAP was already activated 6 h after unilateral ureteral obstruction (UUO). Tubular YAP deficiency greatly attenuated tubular cell overproliferation, tubular injury, and renal inflammation induced by UUO or cisplatin. YAP promoted the transcription of the transcription factor KLF5. Consistent with this, the elevated expression of KLF5 and its target genes in Mst1/2 dKO or UUO kidneys was blocked by ablation of Yap in tubular cells. Inhibition of KLF5 prevented tubular cell overproliferation, tubular injury, and renal inflammation in Mst1/2 dKO kidneys. Therefore, our results demonstrate that tubular YAP is a key player in kidney injury. YAP and KLF5 form a transcriptional cascade, where tubular YAP activation induced by kidney injury promotes KLF5 transcription. Activation of this cascade induces tubular cell overproliferation, tubular injury, and renal inflammation.
Keywords: Hippo; KLF5; MST1; MST2; UUO; YAP; kidney; tubular injury.
Copyright © 2024 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

Similar articles
-
Tubule-Specific Mst1/2 Deficiency Induces CKD via YAP and Non-YAP Mechanisms.J Am Soc Nephrol. 2020 May;31(5):946-961. doi: 10.1681/ASN.2019101052. Epub 2020 Apr 6. J Am Soc Nephrol. 2020. PMID: 32253273 Free PMC article.
-
Targeting mammalian serine/threonine-protein kinase 4 through Yes-associated protein/TEA domain transcription factor-mediated epithelial-mesenchymal transition ameliorates diabetic nephropathy orchestrated renal fibrosis.Metabolism. 2020 Jul;108:154258. doi: 10.1016/j.metabol.2020.154258. Epub 2020 May 3. Metabolism. 2020. PMID: 32376130
-
YAP represses the TEAD-NF-κB complex and inhibits the growth of clear cell renal cell carcinoma.Sci Signal. 2024 Jul 2;17(843):eadk0231. doi: 10.1126/scisignal.adk0231. Epub 2024 Jul 2. Sci Signal. 2024. PMID: 38954637
-
Hippo signaling in the kidney: the good and the bad.Am J Physiol Renal Physiol. 2016 Aug 1;311(2):F241-8. doi: 10.1152/ajprenal.00500.2015. Epub 2016 May 18. Am J Physiol Renal Physiol. 2016. PMID: 27194720 Free PMC article. Review.
-
Hippo-yap signaling in ocular development and disease.Dev Dyn. 2018 Jun;247(6):794-806. doi: 10.1002/dvdy.24628. Epub 2018 Apr 23. Dev Dyn. 2018. PMID: 29532607 Free PMC article. Review.
Cited by
-
Exploring the Functionality of the Krüppel-like Factors in Kidney Development, Metabolism, and Diseases.Life (Basel). 2024 Dec 17;14(12):1671. doi: 10.3390/life14121671. Life (Basel). 2024. PMID: 39768378 Free PMC article. Review.
-
The Role of Yes-Associated Protein in Inflammatory Diseases and Cancer.MedComm (2020). 2025 Mar 10;6(3):e70128. doi: 10.1002/mco2.70128. eCollection 2025 Mar. MedComm (2020). 2025. PMID: 40066231 Free PMC article. Review.
-
An improved TEAD dominant-negative protein inhibitor to study Hippo YAP1/TAZ-dependent transcription.bioRxiv [Preprint]. 2024 Oct 3:2024.10.03.615022. doi: 10.1101/2024.10.03.615022. bioRxiv. 2024. PMID: 39502361 Free PMC article. Preprint.
-
Interstitial Fluid Shear Stress Induces the Synthetic Phenotype Switching of VSMCs to Release Pro-calcified Extracellular Vesicles via EGFR-MAPK-KLF5 Pathway.Int J Biol Sci. 2024 Apr 29;20(7):2727-2747. doi: 10.7150/ijbs.90725. eCollection 2024. Int J Biol Sci. 2024. PMID: 38725857 Free PMC article.
-
Multimodal spatial transcriptomic characterization of mouse kidney injury and repair.Nat Commun. 2025 Aug 14;16(1):7567. doi: 10.1038/s41467-025-62599-9. Nat Commun. 2025. PMID: 40813851 Free PMC article.
References
-
- Humphreys B.D. Mechanisms of Renal Fibrosis. Annu. Rev. Physiol. 2018;80:309–326. - PubMed
-
- Levin A. Global challenges in kidney diseases. Nephrol. Dial. Transpl. 2018;33:371–372. - PubMed
-
- Zhang L., Wang F., Wang L., Wang W., Liu B., Liu J., Chen M., He Q., Liao Y., Yu X., et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379:815–822. - PubMed
-
- Liu B.C., Tang T.T., Lv L.L., Lan H.Y. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int. 2018;93:568–579. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

