Subsequent surgical treatment or maintenance immunotherapy in stage III lung cancer patients achieving a favorable response following neoadjuvant immunotherapy: A matched retrospective cohort study from the surgical perspective
- PMID: 38414317
- PMCID: PMC10995706
- DOI: 10.1111/1759-7714.15247
Subsequent surgical treatment or maintenance immunotherapy in stage III lung cancer patients achieving a favorable response following neoadjuvant immunotherapy: A matched retrospective cohort study from the surgical perspective
Abstract
Background: Current treatment strategies for advanced non-small cell lung cancer (NSCLC) are highly individualized and subject to ongoing debates. In the era of immunotherapy, surgery assumes a critical role. The aim of this study was to investigate if subsequent surgical intervention, following a favorable response to immunotherapy and chemotherapy, could yield a more favorable prognosis for patients with advanced stage III NSCLC compared to the continuation of immunotherapy and chemotherapy.
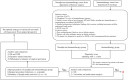
Methods: We included patients whose tumors exhibited a favorable response (including partial response [PR] and complete response [CR]) to immunotherapy and chemotherapy. These patients were categorized into two groups based on their subsequent treatment plans: surgical and nonsurgical (continuation of maintenance immunotherapy and chemotherapy). The efficacy and long-term prognosis of these groups were compared after matching them in a 1:1 ratio using propensity scores.
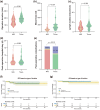
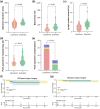
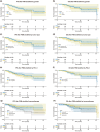
Results: In total, 186 patients (93 in each group) were included in this study after matching via propensity scores. The 1- and 3-year overall survival (OS) and progression-free survival (PFS) rates were 96.0%, 88.5%, and 93.1%, 80.7% in the surgical group, and 93.2%, 83.1%, and 57.7%, 50.4% in the nonsurgical group, respectively. Patients in the surgical group exhibited significantly superior PFS and OS compared to those in the nonsurgical group (p = 0.025 and p = 0.00086). Univariate and multivariate analyses confirmed ΔBMI, Δtumor size reduction, tumor response, earlier clinical stage (IIIb vs. IIIa), and surgery as independent protective factor for patient prognosis. We further selected 101 patients with CR (39 in the surgical group and 62 in the nonsurgical group) and found that patients in the surgical group were significantly better in both PFS and OS. Our subgroup analysis in postoperative patients demonstrated that different surgical strategies did not significantly affect the long-term prognosis of patients (PFS and OS) but could impact their perioperative experience.
Conclusion: Patients with advanced stage III NSCLC, whose tumors achieved PR and CR after 2-4 cycles of immunotherapy combined with chemotherapy, experience a more promising prognosis with subsequent surgical intervention compared with the continued immunotherapy. Despite encountering formidable obstacles, such as protracted surgical procedures and associated trauma, we must rise to the challenge and unleash the power of surgery after immunotherapy in advanced NSCLC.
Keywords: advanced stage III non‐small cell lung cancer; immunotherapy and chemotherapy; promising prognosis; surgery intervention.
© 2024 The Authors. Thoracic Cancer published by John Wiley & Sons Australia, Ltd.
Conflict of interest statement
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication.
Figures



Similar articles
-
Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent.Cochrane Database Syst Rev. 2017 Dec 16;12(12):CD011300. doi: 10.1002/14651858.CD011300.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2021 Dec 6;12:CD011300. doi: 10.1002/14651858.CD011300.pub3. PMID: 29247502 Free PMC article. Updated.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Optimisation of chemotherapy and radiotherapy for untreated Hodgkin lymphoma patients with respect to second malignant neoplasms, overall and progression-free survival: individual participant data analysis.Cochrane Database Syst Rev. 2017 Sep 13;9(9):CD008814. doi: 10.1002/14651858.CD008814.pub2. Cochrane Database Syst Rev. 2017. PMID: 28901021 Free PMC article.
-
Neoadjuvant treatment for stage III and IV cutaneous melanoma.Cochrane Database Syst Rev. 2023 Jan 17;1(1):CD012974. doi: 10.1002/14651858.CD012974.pub2. Cochrane Database Syst Rev. 2023. PMID: 36648215 Free PMC article.
References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. - PubMed
-
- Remon J, Soria JC, Peters S. Early and locally advanced non‐small‐cell lung cancer: an update of the ESMO clinical practice guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol. 2021;32(12):1637–1642. - PubMed
-
- Temel JS, Petrillo LA, Greer JA. Patient‐centered palliative care for patients with advanced lung cancer. J Clin Oncol. 2022;40(6):626–634. - PubMed
-
- Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN guidelines® insights: non‐small cell lung cancer, version 2.2023. J Natl Compr Canc Netw. 2023;21(4):340–350. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

