Natural Products-Based Inhaled Formulations for Treating Pulmonary Diseases
- PMID: 38414528
- PMCID: PMC10898359
- DOI: 10.2147/IJN.S451206
Natural Products-Based Inhaled Formulations for Treating Pulmonary Diseases
Abstract
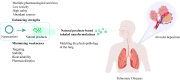
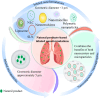
Given the unique physiological and pathological characteristics of the lung, the direct, inhalable route is more conducive to pulmonary drug delivery and disease control than traditional systemic drug delivery, significantly circumventing drug loss, off-target effects, systemic and organ toxicity, etc., and is widely regarded as the preferred regimen for pulmonary drug delivery. However, very few lung diseases are currently treated with the preferred inhaled formulations, such as asthma, chronic obstructive pulmonary disease and pulmonary hypertension. And there is a lack of appropriate inhaled formulations for other critical lung diseases, such as lung cancer and pulmonary fibrosis, due to the fact that the physicochemical properties of the drugs and their pharmacokinetic profiles do not match the physiology of the lung, and conventional inhalation devices are unable to deliver them to the specific parts of the lung. Phytochemicals of natural origin, due to their wide availability and clear safety profile, hold great promise for the preparation of inhalable formulations to improve the current dilemma in the treatment of lung diseases. In particular, the preparation of inhalable formulations based on nano- and microparticulate carriers for drug delivery to deep lung tissues, which overcome the shortcomings of conventional inhalation therapies while targeting the drug activity directly to a specific part of the lung, may be the best approach to change the current dilemma of lung disease treatment. In this review, we discuss recent advances in nano- and micron-carrier-based inhalation formulations for the delivery of natural products for the treatment of pulmonary diseases, which may represent an opportunity for practical clinical translation of natural products.
Keywords: inhalation therapy; inhaled nanoformulations; nanomedicine; natural products; pulmonary diseases.
© 2024 Yong et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Inhaled RNA drugs to treat lung diseases: Disease-related cells and nano-bio interactions.Adv Drug Deliv Rev. 2023 Dec;203:115144. doi: 10.1016/j.addr.2023.115144. Epub 2023 Nov 22. Adv Drug Deliv Rev. 2023. PMID: 37995899 Review.
-
Recent Updates in Inhalable Drug Delivery System against Various Pulmonary Diseases: Challenges and Future Perspectives.Curr Drug Deliv. 2024;21(10):1320-1345. doi: 10.2174/0115672018265571231011093546. Curr Drug Deliv. 2024. PMID: 37870055 Review.
-
Inhaled Formulation Design for the Treatment of Lung Infections.Curr Pharm Des. 2015;21(27):3875-901. doi: 10.2174/1381612821666150820110305. Curr Pharm Des. 2015. PMID: 26290199 Review.
-
[Standard technical specifications for methacholine chloride (Methacholine) bronchial challenge test (2023)].Zhonghua Jie He He Hu Xi Za Zhi. 2024 Feb 12;47(2):101-119. doi: 10.3760/cma.j.cn112147-20231019-00247. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38309959 Chinese.
-
Nano-Strategies for Improving the Bioavailability of Inhaled Pharmaceutical Formulations.Mini Rev Med Chem. 2020;20(13):1258-1271. doi: 10.2174/1389557520666200509235945. Mini Rev Med Chem. 2020. PMID: 32386491 Review.
Cited by
-
The Role of Inhaled Chitosan-Based Nanoparticles in Lung Cancer Therapy.Pharmaceutics. 2024 Jul 23;16(8):969. doi: 10.3390/pharmaceutics16080969. Pharmaceutics. 2024. PMID: 39204314 Free PMC article. Review.
-
Investigation of Factors Influencing the Effectiveness of Deformable Nanovesicles for Insulin Nebulization Inhalation.Pharmaceutics. 2024 Jun 29;16(7):879. doi: 10.3390/pharmaceutics16070879. Pharmaceutics. 2024. PMID: 39065576 Free PMC article.
-
Beyond the pill: incrimination of nuclear factor-kappa B and their targeted phytomedicine for pulmonary fibrosis.Naunyn Schmiedebergs Arch Pharmacol. 2025 Mar 26. doi: 10.1007/s00210-025-04067-1. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40137966 Review.
-
TFR1 as a Biomarker of Pulmonary Fibrosis Development in COPD Patients.Int J Chron Obstruct Pulmon Dis. 2025 Aug 5;20:2715-2725. doi: 10.2147/COPD.S527782. eCollection 2025. Int J Chron Obstruct Pulmon Dis. 2025. PMID: 40786747 Free PMC article.
-
Research advances in polyphenols from Chinese herbal medicine for the prevention and treatment of chronic obstructive pulmonary disease: a review.Naunyn Schmiedebergs Arch Pharmacol. 2025 Aug;398(8):9451-9470. doi: 10.1007/s00210-025-03945-y. Epub 2025 Mar 4. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40035820 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical