Serum urate is associated with an increased risk of inflammatory bowel disease: A bidirectional Mendelian randomization study
- PMID: 38414603
- PMCID: PMC10895627
- DOI: 10.12998/wjcc.v12.i5.891
Serum urate is associated with an increased risk of inflammatory bowel disease: A bidirectional Mendelian randomization study
Abstract
Background: Previous studies have indicated bidirectional associations between urate levels and inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn's disease (CD). However, it remains unclear whether the observations are causal because of confounding factors.
Aim: To investigate the causal associations between urate levels and IBD using bidirectional Mendelian randomization (MR).
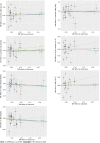
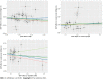
Methods: Independent genetic variants for urate levels and IBD were selected as instrumental variables from published genome-wide association studies (GWASs). Summary statistics for instrument-outcome associations were retrieved from three separate databases for IBD (the UK Biobank, the FinnGen database and a large GWAS meta-analysis) and one for urate levels (a large GWAS meta-analysis). MR analyses included the inverse-variance-weighted method, weighted-median estimator, MR-Egger and sensitivity analyses (MR-PRESSO). A meta-analysis was also conducted to merge the data from separate outcome databases using a fixed-effects model.
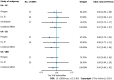
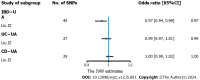
Results: Genetically higher serum urate levels were strongly associated with an increased risk of UC [odds ratio (OR): 1.95, 95% confidence interval (CI): 1.86-2.05] after outlier correction, and the ORs (95%CIs) for IBD and CD were 0.94 (95%CI: 0.86-1.03) and 0.91 (95%CI: 0.80-1.04), respectively. Animal studies have confirmed the positive association between urate levels and UC. Moreover, genetically predicted IBD was inversely related to urate levels (OR: 0.97, 95%CI: 0.94-0.99). However, no association was observed between genetically influenced UC or CD and urate levels.
Conclusion: Urate levels might be risk factors for UC, whereas genetically predicted IBD was inversely associated with urate levels. These findings provide essential new insight for treating and preventing IBD.
Keywords: Antioxidant; Inflammatory bowel disease; Mendelian randomization; Single nucleotide polymorphism; Urate levels.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no conflicts of interest.
Figures


Similar articles
-
Depression and Inflammatory Bowel Disease: A Bidirectional Two-sample Mendelian Randomization Study.J Crohns Colitis. 2022 May 10;16(4):633-642. doi: 10.1093/ecco-jcc/jjab191. J Crohns Colitis. 2022. PMID: 34739073
-
Are neurodegenerative diseases associated with an increased risk of inflammatory bowel disease? A two-sample Mendelian randomization study.Front Immunol. 2022 Sep 8;13:956005. doi: 10.3389/fimmu.2022.956005. eCollection 2022. Front Immunol. 2022. PMID: 36159838 Free PMC article.
-
Causal association between inflammatory bowel disease and IgA nephropathy: A bidirectional two-sample Mendelian randomization study.Front Genet. 2022 Nov 16;13:1002928. doi: 10.3389/fgene.2022.1002928. eCollection 2022. Front Genet. 2022. PMID: 36467999 Free PMC article.
-
Inflammatory bowel disease is causally related to irritable bowel syndrome: a bidirectional two-sample Mendelian randomization study.Front Med (Lausanne). 2023 Apr 17;10:1166683. doi: 10.3389/fmed.2023.1166683. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37138734 Free PMC article.
-
Causality of inflammatory bowel disease and seborrheic keratosis: A bidirectional two-sample Mendelian randomization study.Skin Res Technol. 2024 Aug;30(8):e13876. doi: 10.1111/srt.13876. Skin Res Technol. 2024. PMID: 39081143 Free PMC article.
References
-
- Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–2778. - PubMed
-
- Benchimol EI, Guttmann A, Griffiths AM, Rabeneck L, Mack DR, Brill H, Howard J, Guan J, To T. Increasing incidence of paediatric inflammatory bowel disease in Ontario, Canada: evidence from health administrative data. Gut. 2009;58:1490–1497. - PubMed
-
- Frolkis AD, Dykeman J, Negrón ME, Debruyn J, Jette N, Fiest KM, Frolkis T, Barkema HW, Rioux KP, Panaccione R, Ghosh S, Wiebe S, Kaplan GG. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996–1006. - PubMed
-
- Huang H, Fang M, Jostins L, Umićević Mirkov M, Boucher G, Anderson CA, Andersen V, Cleynen I, Cortes A, Crins F, D'Amato M, Deffontaine V, Dmitrieva J, Docampo E, Elansary M, Farh KK, Franke A, Gori AS, Goyette P, Halfvarson J, Haritunians T, Knight J, Lawrance IC, Lees CW, Louis E, Mariman R, Meuwissen T, Mni M, Momozawa Y, Parkes M, Spain SL, Théâtre E, Trynka G, Satsangi J, van Sommeren S, Vermeire S, Xavier RJ International Inflammatory Bowel Disease Genetics Consortium, Weersma RK, Duerr RH, Mathew CG, Rioux JD, McGovern DPB, Cho JH, Georges M, Daly MJ, Barrett JC. Fine-mapping inflammatory bowel disease loci to single-variant resolution. Nature. 2017;547:173–178. - PMC - PubMed
LinkOut - more resources
Full Text Sources

