The dose-response relationship of subretinal gene therapy with rAAV2tYF-CB-h RS1 in a mouse model of X-linked retinoschisis
- PMID: 38414621
- PMCID: PMC10898246
- DOI: 10.3389/fmed.2024.1304819
The dose-response relationship of subretinal gene therapy with rAAV2tYF-CB-h RS1 in a mouse model of X-linked retinoschisis
Abstract
Purpose: X-linked retinoschisis (XLRS), due to loss-of-function mutations in the retinoschisin (RS1) gene, is characterized by a modest to severe decrease in visual acuity. Clinical trials for XLRS utilizing intravitreal (IVT) gene therapy showed ocular inflammation. We conducted a subretinal dose-response preclinical study using rAAV2tYF-CB-hRS1 utilizing the Rs1 knockout (Rs1-KO) mouse to investigate short- and long-term retinal rescue after subretinal gene delivery.
Methods: Rs1-KO mice were subretinally injected with 2 μL of rAAV2tYF-CB-hRS1 vector with 8E9 viral genomes (vg)/eye, 8E8 vg/eye, 8E7 vg/eye, or sham injection, and compared to untreated eyes. Reconstitution of human RS1 protein was detected using western blotting. Analysis of retinal function by electroretinography (ERG) and structural analysis by optical coherence tomography (OCT) were performed at 1, 2, 3, 5, 7, and 12 months post injection (MPI). Immunohistochemistry (IHC) was performed to evaluate cone rescue on the cellular level. Functional vision was evaluated using a visually guided swim assay (VGSA).
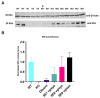
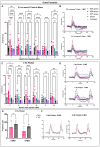
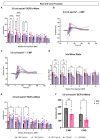
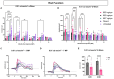
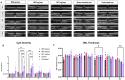
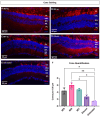
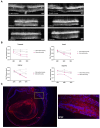
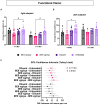
Results: Western blotting analysis showed human RS1 protein expression in a dose-dependent manner. Quantification of western blotting showed that the RS1 protein expression in mice treated with the 8E8 vg dose was near the wild-type (WT) expression levels. ERG demonstrated dose-dependent effects: At 1 MPI the 8E8 vg dose treated eyes had higher light-adapted (LA) ERG amplitudes in 3.0 flash and 5 Hz flicker compared to untreated (p < 0.0001) and sham-treated eyes (p < 0.0001) which persisted until the 12 MPI endpoint, consistent with improved cone function. ERG b-wave amplitudes were higher in response to dark-adapted (DA) 0.01 dim flash and 3.0 standard combined response (SCR) compared to sham-treated (p < 0.01) and untreated eyes (p < 0.001) which persisted until 3 MPI, suggesting short-term improvement of the rod photoreceptors. All injections, including sham-treated, resulted in a cyst severity score of 1 (no cavities), with significant reductions compared to untreated eyes up to 3 MPI (p < 0.05). The high and low dose groups showed inconsistent ERG improvements, despite reduced cyst severity, emphasizing the dose-dependent nature of gene augmentation's efficacy and the tenuous connection between cyst reduction and ERG improvement. IHC data showed a significant cone rescue in eyes treated with the 8E8 vg dose compared to sham-treated and untreated eyes. VGSA showed better functional vision in 8E8 vg dose treated mice. Eyes treated with the highest dose showed occasional localized degeneration in the outer nuclear layer.
Conclusion: Our data suggest that a dose of 8E8 vg/eye subretinally improves retinal function and structure in the Rs1-KO mouse. It improves cone function, rod function, and reduces cyst severity. Sham treatment resolves schisis cysts, but 8E8 vg/eye is needed for optimal retinal electrical function rescue. These findings offer a promising path for clinical translation to human trials.
Keywords: Rs1 knockout mouse; X-linked retinoschisis; dose-response; electroretinogram; functional vision; subretinal gene therapy; visually guided swim assay.
Copyright © 2024 Hassan, Hsu, Thompson, Kalmanek, VandeLune, Stanley and Drack.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
An osmolarity dependent mechanism partially ameliorates retinal cysts and rescues cone function in a mouse model of X-linked retinoschisis.Front Med (Lausanne). 2024 Oct 15;11:1302119. doi: 10.3389/fmed.2024.1302119. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39473494 Free PMC article.
-
Preclinical Dose-Escalation Study of Intravitreal AAV-RS1 Gene Therapy in a Mouse Model of X-linked Retinoschisis: Dose-Dependent Expression and Improved Retinal Structure and Function.Hum Gene Ther. 2016 May;27(5):376-89. doi: 10.1089/hum.2015.142. Hum Gene Ther. 2016. PMID: 27036983 Free PMC article.
-
AAV2/4-RS1 gene therapy in the retinoschisin knockout mouse model of X-linked retinoschisis.PLoS One. 2022 Dec 7;17(12):e0276298. doi: 10.1371/journal.pone.0276298. eCollection 2022. PLoS One. 2022. PMID: 36477475 Free PMC article.
-
Of men and mice: Human X-linked retinoschisis and fidelity in mouse modeling.Prog Retin Eye Res. 2022 Mar;87:100999. doi: 10.1016/j.preteyeres.2021.100999. Epub 2021 Aug 11. Prog Retin Eye Res. 2022. PMID: 34390869 Review.
-
X-linked juvenile retinoschisis: clinical diagnosis, genetic analysis, and molecular mechanisms.Prog Retin Eye Res. 2012 May;31(3):195-212. doi: 10.1016/j.preteyeres.2011.12.002. Epub 2012 Jan 3. Prog Retin Eye Res. 2012. PMID: 22245536 Free PMC article. Review.
Cited by
-
An osmolarity dependent mechanism partially ameliorates retinal cysts and rescues cone function in a mouse model of X-linked retinoschisis.Front Med (Lausanne). 2024 Oct 15;11:1302119. doi: 10.3389/fmed.2024.1302119. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39473494 Free PMC article.
-
Immune landscape in children with X-linked retinoschisis.BMC Immunol. 2025 Aug 8;26(1):59. doi: 10.1186/s12865-025-00741-4. BMC Immunol. 2025. PMID: 40781274 Free PMC article.
References
-
- Vijayasarathy C, Zeng Y, Brooks MJ, Fariss RN, Sieving PA. Genetic rescue of X-linked retinoschisis mouse (Rs1(−/y)) retina induces quiescence of the retinal microglial inflammatory state following AAV8-RS1 gene transfer and identifies gene networks underlying retinal recovery. Hum Gene Ther. (2021) 32:667–81. doi: 10.1089/hum.2020.213, PMID: - DOI - PMC - PubMed
-
- Ye GJ, Budzynski E, Sonnentag P, Miller PE, Sharma AK, Ver Hoeve JN, et al. . Safety and biodistribution evaluation in cynomolgus macaques of rAAV2tYF-CB-hRS1, a recombinant adeno-associated virus vector expressing retinoschisin. Hum Gene Ther Clin Dev. (2015) 26:165–76. doi: 10.1089/humc.2015.076, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

