Establishment of new transurethral catheterization methods for male mice
- PMID: 38414648
- PMCID: PMC10898326
- DOI: 10.1093/biomethods/bpae005
Establishment of new transurethral catheterization methods for male mice
Abstract
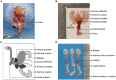
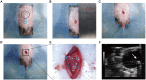
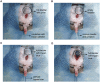
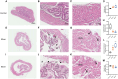
Transurethral catheterization in mice is multifaceted, serving essential functions such as perfusion and drug delivery, and is critical in the development of various urological animal disease models. The complex anatomy of the male mouse urethra presents significant challenges in transurethral catheterization, leading to a predominance of research focused on female specimens. This bias limits the utilization of male mice in lower urinary tract disease studies. Our research aims to develop new reliable methods for transurethral catheterization in adult male mice, thereby expanding their use in relevant disease research. Experiments were conducted on adult male C57BL/6J mice. Utilizing a PE10 catheter measuring 4.5-5 cm in length, the catheter was inserted into the bladder via the mouse's urethra under anesthesia. The intubation technique entailed regulating the insertion force, ensuring the catheter's lubrication, using a trocar catheter, modifying the catheter's trajectory, and accommodating the curvature of the bladder neck. Post-catheter insertion, ultrasound imaging was employed to confirm the catheter's accurate positioning within the bladder. Subsequent to catheterization, the bladder was perfused using trypan blue. This method was further validated through its successful application in establishing an acute urinary retention (AUR) model, where the mouse bladder was infused with saline to a pressure of 50 or 80 cm H2O, maintained steadily for 30 min. A thorough morphological assessment of the mouse bladder was conducted after the infusion. Our study successfully pioneered methods for transurethral catheterization in male mice. This technique not only facilitates precise transurethral catheterization but also proves applicable to male mouse models for lower urinary tract diseases, such as AUR.
Keywords: bladder; bladder perfusion; lower urinary tract; male mouse; transurethral catheterization.
© The Author(s) 2024. Published by Oxford University Press.
Figures


Similar articles
-
Novel catheter design enables transurethral catheterization of male mice.Am J Physiol Renal Physiol. 2020 Jul 1;319(1):F29-F32. doi: 10.1152/ajprenal.00121.2020. Epub 2020 May 28. Am J Physiol Renal Physiol. 2020. PMID: 32463724 Free PMC article.
-
Transurethral bladder catheterization of male rhesus macaques: a refinement of approach.J Med Primatol. 2011 Oct;40(5):342-50. doi: 10.1111/j.1600-0684.2011.00494.x. J Med Primatol. 2011. PMID: 21950722
-
Acute urinary retention and subsequent catheterization cause lipid peroxidation and oxidative DNA damage in the bladder: preventive effect of edaravone, a free-radical scavenger.BJU Int. 2009 Sep;104(5):713-7. doi: 10.1111/j.1464-410X.2009.08471.x. Epub 2009 Mar 30. BJU Int. 2009. PMID: 19338546
-
[Acute urinary retention: a few simple rules for a successful catheterization].Ther Umsch. 2015 Jan;72(1):39-42. doi: 10.1024/0040-5930/a000636. Ther Umsch. 2015. PMID: 25533254 Review. German.
-
The Reten-World survey of the management of acute urinary retention: preliminary results.BJU Int. 2008 Mar;101 Suppl 3:27-32. doi: 10.1111/j.1464-410X.2008.07491.x. BJU Int. 2008. PMID: 18307683 Review.
References
-
- Waterston RH, Lindblad-Toh K, Birney E, et al.Initial sequencing and comparative analysis of the mouse genome. Nature 2002;420:520–62. - PubMed
-
- Van Den Eeden SK, Sarma AV, Rutledge BN, et al.Effect of intensive glycemic control and diabetes complications on lower urinary tract symptoms in men with type 1 diabetes: diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes Care 2009;32:664–70. - PMC - PubMed
-
- Hooton TM, Stamm WE.. Diagnosis and treatment of uncomplicated urinary tract infection. Infect Dis Clin North Am 1997;11:551–81. - PubMed
LinkOut - more resources
Full Text Sources
