Anti‑osteoclastogenic effect of fermented mealworm extract by inhibiting RANKL‑induced NFATc1 action
- PMID: 38414787
- PMCID: PMC10895579
- DOI: 10.3892/etm.2024.12418
Anti‑osteoclastogenic effect of fermented mealworm extract by inhibiting RANKL‑induced NFATc1 action
Abstract
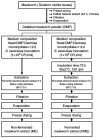
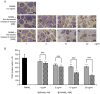
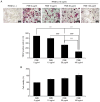
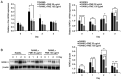
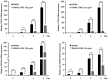
Augmented osteoclast activity and differentiation can lead to destructive bone diseases, such as arthritis and osteoporosis. Therefore, modulating osteoclastogenesis and differentiation may serve to be a possible strategy for treating such diseases. Tenebrio molitor larvae, also known as mealworms, are considered a good source of protein with nutritional value, digestibility, flavor and functional properties, such as antioxidant, anti-diabetic and anti-obesity effects. However, the role of mealworms in osteoclastogenesis remains poorly understood. The present study therefore investigated the effects of fermented mealworm extract (FME) on receptor activator of nuclear factor κB ligand (RANKL)-induced osteoclastogenesis in bone marrow-derived macrophages (BMMs) whilst also attempting to understand the underlying mechanism, if any. The cells treated with RANKL were used as the negative control. To prepare FME, defatted mealworm powder was fermented with a Saccharomyces cerevisiae strain, and then extracted with fermented alcohol. Cell viability of BMMs isolated from 5-week-old Institute of Cancer Research mice was measured using Cell Counting Kit-8 assay. Subsequently, the effects of FME on osteoclast differentiation were measured using tartrate-resistant acid phosphatase (TRAP) staining. In addition, expression of markers associated with osteoclast differentiation was assessed by reverse transcription-quantitative PCR. Expression of nuclear factor of activated T-cells cytoplasmic 1 (NFATc1) was assessed by western blotting. TRAP staining revealed that FME inhibited osteoclast differentiation in a dose-dependent manner (10-100 µg/ml) without causing cytotoxicity. Specifically, the formation of osteoclasts appear to have been suppressed by FME as indicated by the reduction in the number of TRAP-positive multinucleated cells observed. Furthermore, FME treatment significantly decreased the mRNA expression of c-Fos, whilst also significantly decreasing the expression of NFATc1 on both protein and mRNA levels. c-Fos and NFATc1 are transcription factors that can regulate osteoclast differentiation. FME treatment also reduced the expression of genes associated with osteoclast differentiation and function, including dendritic cell-specific transmembrane protein, osteoclast associated Ig-like receptor, Cathepsin K and TRAP, compared with that in the control group. Subsequently, FME was found to effectively suppress RANKL-induced osteoclast differentiation compared with that by the non-fermented mealworm extract. These findings suggest that FME may confer anti-osteoclastogenic effects, providing insights into its potential application in treatment of osteoporosis.
Keywords: cytoplasmic 1; fermentation; mealworm; nuclear factor of activated T-cells; osteoclastogenesis; receptor activator of NF-κB ligand.
Copyright: © Ham and Lee.
Conflict of interest statement
The authors declared that they have no competing interests.
Figures
Similar articles
-
Isofraxidin Inhibits Receptor Activator of Nuclear Factor-κB Ligand-Induced Osteoclastogenesis in Bone Marrow-Derived Macrophages Isolated from Sprague-Dawley Rats by Regulating NF-κB/NFATc1 and Akt/NFATc1 Signaling Pathways.Cell Transplant. 2021 Jan-Dec;30:963689721990321. doi: 10.1177/0963689721990321. Cell Transplant. 2021. PMID: 33573387 Free PMC article.
-
Caffeic acid 3,4-dihydroxy-phenethyl ester suppresses receptor activator of NF-κB ligand–induced osteoclastogenesis and prevents ovariectomy-induced bone loss through inhibition of mitogen-activated protein kinase/activator protein 1 and Ca2+–nuclear factor of activated T-cells cytoplasmic 1 signaling pathways.J Bone Miner Res. 2012 Jun;27(6):1298-1308. doi: 10.1002/jbmr.1576. J Bone Miner Res. 2012. PMID: 22337253
-
Interleukin 29 inhibits RANKL-induced osteoclastogenesis via activation of JNK and STAT, and inhibition of NF-κB and NFATc1.Cytokine. 2019 Jan;113:144-154. doi: 10.1016/j.cyto.2018.06.032. Epub 2018 Jul 9. Cytokine. 2019. PMID: 30001863
-
Inhibition of receptor activator of nuclear factor-κB ligand- or lipopolysaccharide-induced osteoclast formation by conophylline through downregulation of CREB.Immunol Lett. 2014 Sep;161(1):31-7. doi: 10.1016/j.imlet.2014.04.006. Epub 2014 May 2. Immunol Lett. 2014. PMID: 24792671
-
Agelasine D suppresses RANKL-induced osteoclastogenesis via down-regulation of c-Fos, NFATc1 and NF-κB.Mar Drugs. 2014 Nov 24;12(11):5643-56. doi: 10.3390/md12115643. Mar Drugs. 2014. PMID: 25421321 Free PMC article.
Cited by
-
PTHrP participates in the bone destruction of middle ear cholesteatoma via promoting macrophage differentiation into osteoclasts induced by RANKL.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024 May 28;49(5):655-666. doi: 10.11817/j.issn.1672-7347.2024.230482. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 39174879 Free PMC article. Chinese, English.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
