Anisotropic microtopography surface of chitosan scaffold regulating skin precursor-derived Schwann cells towards repair phenotype promotes neural regeneration
- PMID: 38414797
- PMCID: PMC10898340
- DOI: 10.1093/rb/rbae005
Anisotropic microtopography surface of chitosan scaffold regulating skin precursor-derived Schwann cells towards repair phenotype promotes neural regeneration
Abstract
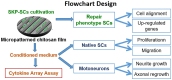
For repairing peripheral nerve and spinal cord defects, biomaterial scaffold-based cell-therapy was emerged as an effective strategy, requiring the positive response of seed cells to biomaterial substrate and environment signals. Previous work highlighted that the imposed surface properties of scaffold could provide important guidance cues to adhered cells for polarization. However, the insufficiency of native Schwann cells and unclear cellular response mechanisms remained to be addressed. Given that, this study aimed to illuminate the micropatterned chitosan-film action on the rat skin precursor-derived Schwann cells (SKP-SCs). Chitosan-film with different ridge/groove size was fabricated and applied for the SKP-SCs induction. Results indicated that SKP-SCs cultured on 30 μm size microgroove surface showed better oriented alignment phenotype. Induced SKP-SCs presented similar genic phenotype as repair Schwann cells, increasing expression of c-Jun, neural cell adhesion molecule, and neurotrophic receptor p75. Moreover, SKP-SC-secretome was subjected to cytokine array GS67 assay, data indicated the regulation of paracrine phenotype, a panel of cytokines was verified up-regulated at secreted level and gene expression level in induced SKP-SCs. These up-regulated cytokines exhibit a series of promotive neural regeneration functions, including cell survival, cell migration, cell proliferation, angiogenesis, axon growth, and cellular organization etc. through bioinformatics analysis. Furthermore, the effectively polarized SKP-SCs-sourced secretome, promoted the proliferation and migration capacity of the primarily cultured native rat Schwann cells, and augmented neurites growth of the cultured motoneurons, as well as boosted axonal regrowth of the axotomy-injured motoneurons. Taken together, SKP-SCs obtained pro-neuroregeneration phenotype in adaptive response to the anisotropic topography surface of chitosan-film, displayed the oriented parallel growth, the transition towards repair Schwann cell genic phenotype, and the enhanced paracrine effect on neural regeneration. This study provided novel insights into the potency of anisotropic microtopography surface to Schwann-like cells phenotype regulation, that facilitating to provide promising engineered cell-scaffold in neural injury therapies.
Keywords: anisotropic microtopography; chitosan-film; neural regeneration; phenotype regulation; skin precursor-derived Schwann cells.
© The Author(s) 2024. Published by Oxford University Press.
Figures








Similar articles
-
Promotive effect of skin precursor-derived Schwann cells on brachial plexus neurotomy and motor neuron damage repair through milieu-regulating secretome.Regen Ther. 2024 Apr 22;27:365-380. doi: 10.1016/j.reth.2024.04.002. eCollection 2024 Dec. Regen Ther. 2024. PMID: 38694448 Free PMC article.
-
Extracellular vesicles from skin precursor-derived Schwann cells promote axonal outgrowth and regeneration of motoneurons via Akt/mTOR/p70S6K pathway.Ann Transl Med. 2020 Dec;8(24):1640. doi: 10.21037/atm-20-5965. Ann Transl Med. 2020. PMID: 33490152 Free PMC article.
-
Skin derived precursor Schwann cell-generated acellular matrix modified chitosan/silk scaffolds for bridging rat sciatic nerve gap.Neurosci Res. 2018 Oct;135:21-31. doi: 10.1016/j.neures.2017.12.007. Epub 2017 Dec 27. Neurosci Res. 2018. PMID: 29288689
-
Biomaterial-Based Schwann Cell Transplantation and Schwann Cell-Derived Biomaterials for Nerve Regeneration.Front Cell Neurosci. 2022 Jun 28;16:926222. doi: 10.3389/fncel.2022.926222. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35836742 Free PMC article. Review.
-
New insights into peripheral nerve regeneration: The role of secretomes.Exp Neurol. 2022 Aug;354:114069. doi: 10.1016/j.expneurol.2022.114069. Epub 2022 Apr 6. Exp Neurol. 2022. PMID: 35398149 Review.
Cited by
-
Analytical methods in studying cell force sensing: principles, current technologies and perspectives.Regen Biomater. 2025 Mar 20;12:rbaf007. doi: 10.1093/rb/rbaf007. eCollection 2025. Regen Biomater. 2025. PMID: 40337625 Free PMC article. Review.
-
Biodegradable microspheres via orally deliver celastrol with ameliorated neuropathic pain in diabetes rats.Regen Biomater. 2024 Jul 17;11:rbae087. doi: 10.1093/rb/rbae087. eCollection 2024. Regen Biomater. 2024. PMID: 39055304 Free PMC article.
-
Advances in biomaterial-based tissue engineering for peripheral nerve injury repair.Bioact Mater. 2024 Dec 13;46:150-172. doi: 10.1016/j.bioactmat.2024.12.005. eCollection 2025 Apr. Bioact Mater. 2024. PMID: 39760068 Free PMC article. Review.
-
Biomaterials for neuroengineering: applications and challenges.Regen Biomater. 2025 Feb 21;12:rbae137. doi: 10.1093/rb/rbae137. eCollection 2025. Regen Biomater. 2025. PMID: 40007617 Free PMC article. Review.
-
Unveiling the molecular blueprint of SKP-SCs-mediated tissue engineering-enhanced neuroregeneration.J Nanobiotechnology. 2024 Dec 26;22(1):796. doi: 10.1186/s12951-024-03076-1. J Nanobiotechnology. 2024. PMID: 39725969 Free PMC article.
References
-
- Kuffler DP. An assessment of current techniques for inducing axon regeneration and neurological recovery following peripheral nerve trauma. Prog Neurobiol 2014;116:1–12. - PubMed
-
- Silva NA, Sousa N, Reis RL, Salgado AJ.. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol 2014;114:25–57. - PubMed
-
- New PW, Baxter D, Farry A, Noonan VK.. Estimating the incidence and prevalence of traumatic spinal cord injury in Australia. Arch Phys Med Rehabil 2015;96:76–83. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

