Microbiome-friendly PS/PVP electrospun fibrous membrane with antibiofilm properties for dental engineering
- PMID: 38414799
- PMCID: PMC10898674
- DOI: 10.1093/rb/rbae011
Microbiome-friendly PS/PVP electrospun fibrous membrane with antibiofilm properties for dental engineering
Abstract
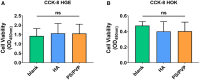
Dental caries is one of the most prevalent and biofilm-associated oral diseases in humans. Streptococcus mutans, with a high ability to form biofilms by adhering to hard surfaces, has been established as an important etiological agent for dental caries. Therefore, it is crucial to find a way to prevent the formation of cariogenic biofilm. Here, we report an electrospun fibrous membrane that could inhibit the adhesion and biofilm formation of S. mutans. Also, the polystyrene (PS)/polyvinyl pyrrolidone (PVP) electrospun fibrous membrane altered the 3D biofilm architecture and decreased water-insoluble extracellular polysaccharide production. Notably, the anti-adhesion mechanism which laid in Coulomb repulsion between the negatively charged PS/PVP electrospun fibrous membrane and S. mutans was detected by zeta potential. Furthermore, metagenomics sequencing analysis and CCK-8 assay indicated that PS/PVP electrospun fibrous membrane was microbiome-friendly and displayed no influence on the cell viability of human gingival epithelial cells and human oral keratinocytes. Moreover, an in vitro simulation experiment demonstrated that PS/PVP electrospun fibrous membrane could decrease colony-forming unit counts of S. mutans effectively, and PS/PVP electrospun fibrous membrane carrying calcium fluoride displayed better anti-adhesion ability than that of PS/PVP electrospun fibrous membrane alone. Collectively, this research showed that the PS/PVP electrospun fibrous membrane has potential applications in controlling and preventing dental caries.
Keywords: Coulomb repulsion; PS/PVP electrospun fibrous membrane; Streptococcus mutans; anti-adhesion; biofilm(s).
© The Author(s) 2024. Published by Oxford University Press.
Figures






References
-
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet 2017;390:1211–59. - PMC - PubMed
-
- Takahashi N, Nyvad B.. Caries ecology revisited: microbial dynamics and the caries process. Caries Res 2008;42:409–18. - PubMed
LinkOut - more resources
Full Text Sources

