The heterogeneity effect of surveillance intervals on progression free survival
- PMID: 38414801
- PMCID: PMC10896158
- DOI: 10.1080/02664763.2022.2145272
The heterogeneity effect of surveillance intervals on progression free survival
Abstract
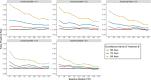
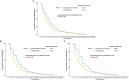
Progression-free survival (PFS) is an increasingly important surrogate endpoint in cancer clinical trials. However, the true time of progression is typically unknown if the evaluation of progression status is only scheduled at given surveillance intervals. In addition, comparison between treatment arms under different surveillance schema is not uncommon. Our aim is to explore whether the heterogeneity of the surveillance intervals may interfere with the validity of the conclusion of efficacy based on PFS, and the extent to which the variation would bias the results. We conduct comprehensive simulation studies to explore the aforementioned goals in a two-arm randomized control trial. We introduce three steps to simulate survival data with predefined surveillance intervals under different censoring rate considerations. We report the estimated hazard ratios and examine false positive rate, power and bias under different surveillance intervals, given different baseline median PFS, hazard ratio and censoring rate settings. Results show that larger heterogeneous lengths of surveillance intervals lead to higher false positive rate and overestimate the power, and the effect of the heterogeneous surveillance intervals may depend upon both the life expectancy of the tumor prognoses and the censoring proportion of the survival data. We also demonstrate such heterogeneity effect of surveillance intervals on PFS in a phase III metastatic colorectal cancer trial. In our opinions, adherence to consistent surveillance intervals should be favored in designing the comparative trials. Otherwise, it needs to be appropriately taken into account when analyzing data.
Keywords: Progression-free survival; cancer clinical trial; false positive rate; power; surveillance interval.
© 2022 Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
This publication is based on research using information obtained from https://data.ProjectDataSphere.org, which is maintained by Project Data Sphere. Neither Project Data Sphere nor the owner(s) of any information from the web site have contributed to, approved or are in any way responsible for the contents of this publication. No potential conflict of interest was reported by the author(s).
Figures




Similar articles
-
Statistical considerations and endpoints for clinical lung cancer studies: Can progression free survival (PFS) substitute overall survival (OS) as a valid endpoint in clinical trials for advanced non-small-cell lung cancer?Transl Lung Cancer Res. 2012 Mar;1(1):26-35. doi: 10.3978/j.issn.2218-6751.2011.12.08. Transl Lung Cancer Res. 2012. PMID: 25806152 Free PMC article. Review.
-
When you look matters: the effect of assessment schedule on progression-free survival.J Natl Cancer Inst. 2007 Mar 21;99(6):428-32. doi: 10.1093/jnci/djk091. J Natl Cancer Inst. 2007. PMID: 17374832
-
The case against censoring of progression-free survival in cancer clinical trials - A pandemic shutdown as an illustration.BMC Med Res Methodol. 2022 Oct 5;22(1):260. doi: 10.1186/s12874-022-01731-5. BMC Med Res Methodol. 2022. PMID: 36199019 Free PMC article.
-
Progression-free survival estimates are shaped by specific censoring rules: Implications for PFS as an endpoint in cancer randomized trials.Eur J Cancer. 2024 May;202:114022. doi: 10.1016/j.ejca.2024.114022. Epub 2024 Mar 20. Eur J Cancer. 2024. PMID: 38547775
-
A Survey of Survival Outcomes for Targeted Cancer Drugs Approved by the US Food and Drug Administration.Ther Innov Regul Sci. 2021 Jul;55(4):676-684. doi: 10.1007/s43441-021-00264-1. Epub 2021 Mar 8. Ther Innov Regul Sci. 2021. PMID: 33683659 Review.
References
-
- Amado R.G., Wolf M., Peeters M., Van Cutsem E., Siena S., Freeman D.J., Juan T., Sikorski R., Suggs S., Radinsky R., Patterson S.D., and Chang D.D., Wild-type kras is required for panitumumab efficacy in patients with metastatic colorectal cancer, J. Clin. Oncol. 26 (2008), pp. 1626–1634. - PubMed
-
- Beaver J.A., Howie L.J., Pelosof L., Kim T., Liu J., Goldberg K.B., Sridhara R., Blumenthal G.M., Farrell A.T., Keegan P., Pazdur R., and Kluetz P.G., A 25-year experience of us food and drug administration accelerated approval of malignant hematology and oncology drugs and biologics a review, JAMA. Oncol. 4 (2018), pp. 849–856. - PubMed
-
- Bender R., Augustin T., and Blettner M., Generating survival times to simulate Cox proportional hazards models, Stat. Med. 24 (2005), pp. 1713–1723. - PubMed
-
- Bogaerts K., Komárek A., and Lesaffre E., Survival Analysis with Interval-Censored Data: A Practical Approach with Examples in R, SAS, and BUGS, Chapman and Hall/CRC, Boca Raton, 2017.
LinkOut - more resources
Full Text Sources
