Axon-like aligned conductive CNT/GelMA hydrogel fibers combined with electrical stimulation for spinal cord injury recovery
- PMID: 38414842
- PMCID: PMC10897856
- DOI: 10.1016/j.bioactmat.2024.01.021
Axon-like aligned conductive CNT/GelMA hydrogel fibers combined with electrical stimulation for spinal cord injury recovery
Abstract
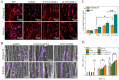
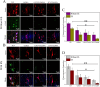
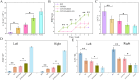
Rehabilitation and regenerative medicine are two promising approaches for spinal cord injury (SCI) recovery, but their combination has been limited. Conductive biomaterials could bridge regenerative scaffolds with electrical stimulation by inducing axon regeneration and supporting physiological electrical signal transmission. Here, we developed aligned conductive hydrogel fibers by incorporating carbon nanotubes (CNTs) into methacrylate acylated gelatin (GelMA) hydrogel via rotating liquid bath electrospinning. The electrospun CNT/GelMA hydrogel fibers mimicked the micro-scale aligned structure, conductivity, and soft mechanical properties of neural axons. For in vitro studies, CNT/GelMA hydrogel fibers supported PC12 cell proliferation and aligned adhesion, which was enhanced by electrical stimulation (ES). Similarly, the combination of aligned CNT/GelMA hydrogel fibers and ES promoted neuronal differentiation and axon-like neurite sprouting in neural stem cells (NSCs). Furthermore, CNT/GelMA hydrogel fibers were transplanted into a T9 transection rat spinal cord injury model for in vivo studies. The results showed that the incorporating CNTs could remain at the injury site with the GelMA fibers biodegraded and improve the conductivity of regenerative tissue. The aligned structure of the hydrogel could induce the neural fibers regeneration, and the ES enhanced the remyelination and axonal regeneration. Behavioral assessments and electrophysiological results suggest that the combination of aligned CNT/GelMA hydrogel fibers and ES could significantly restore motor function in rats. This study demonstrates that conductive aligned CNT/GelMA hydrogel fibers can not only induce neural regeneration as a scaffold but also support ESto promote spinal cord injury recovery. The conductive hydrogel fibers enable merging regenerative medicine and rehabilitation, showing great potential for satisfactory locomotor recovery after SCI.
Keywords: CNT/GelMA; Conductive hydrogel fibers; Electrical stimulation; NSCs differentiation; Spinal cord injury.
© 2024 The Authors.
Conflict of interest statement
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.
Figures







Similar articles
-
Hybrid hydrogel-aligned carbon nanotube scaffolds to enhance cardiac differentiation of embryoid bodies.Acta Biomater. 2016 Feb;31:134-143. doi: 10.1016/j.actbio.2015.11.047. Epub 2015 Nov 24. Acta Biomater. 2016. PMID: 26621696
-
Combination therapy of fingolimod-GelMA hydrogel loaded with neural stem cells and electrical stimulation promotes recovery of spinal cord injury.J Biomater Sci Polym Ed. 2025 Aug 5:1-22. doi: 10.1080/09205063.2025.2541973. Online ahead of print. J Biomater Sci Polym Ed. 2025. PMID: 40763091
-
Injectable Hydrogel Loaded with CDs and FTY720 Combined with Neural Stem Cells for the Treatment of Spinal Cord Injury.Int J Nanomedicine. 2024 May 8;19:4081-4101. doi: 10.2147/IJN.S448962. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38736654 Free PMC article.
-
Regenerative rehabilitation with conductive biomaterials for spinal cord injury.Acta Biomater. 2022 Feb;139:43-64. doi: 10.1016/j.actbio.2020.12.021. Epub 2020 Dec 14. Acta Biomater. 2022. PMID: 33326879 Review.
-
Advances in Conductive Hydrogel for Spinal Cord Injury Repair and Regeneration.Int J Nanomedicine. 2023 Dec 6;18:7305-7333. doi: 10.2147/IJN.S436111. eCollection 2023. Int J Nanomedicine. 2023. PMID: 38084124 Free PMC article. Review.
Cited by
-
Introductory Review of Soft Implantable Bioelectronics Using Conductive and Functional Hydrogels and Hydrogel Nanocomposites.Gels. 2024 Sep 25;10(10):614. doi: 10.3390/gels10100614. Gels. 2024. PMID: 39451267 Free PMC article. Review.
-
Anisotropic structure of nanofiber hydrogel accelerates diabetic wound healing via triadic synergy of immune-angiogenic-neurogenic microenvironments.Bioact Mater. 2025 Jan 11;47:64-82. doi: 10.1016/j.bioactmat.2025.01.004. eCollection 2025 May. Bioact Mater. 2025. PMID: 39877154 Free PMC article.
-
3D bioprinted dynamic bioactive living construct enhances mechanotransduction-assisted rapid neural network self-organization for spinal cord injury repair.Bioact Mater. 2025 Jan 8;46:531-554. doi: 10.1016/j.bioactmat.2024.12.028. eCollection 2025 Apr. Bioact Mater. 2025. PMID: 39886605 Free PMC article.
-
Composite barrier membrane for bone regeneration: advancing biomaterial strategies in defect repair.RSC Adv. 2025 Jan 15;15(2):1290-1299. doi: 10.1039/d4ra07623k. eCollection 2025 Jan 9. RSC Adv. 2025. PMID: 39816171 Free PMC article.
-
Spinal cord injury repair based on drug and cell delivery: From remodeling microenvironment to relay connection formation.Mater Today Bio. 2025 Feb 4;31:101556. doi: 10.1016/j.mtbio.2025.101556. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40026622 Free PMC article. Review.
References
-
- Kiyotake E.A., Martin M.D., Detamore M.S. Regenerative rehabilitation with conductive biomaterials for spinal cord injury. Acta Biomater. 2022;139:43–64. - PubMed
-
- Xiao L., Xie P., Ma J., Shi K., Dai Y., Pang M., Luo J., Tan Z., Ma Y., Wang X., Rong L., He L. A bioinspired injectable, adhesive, and self-healing hydrogel with dual hybrid network for neural regeneration after spinal cord injury. Adv. Mater. 2023;35(41) - PubMed
LinkOut - more resources
Full Text Sources

