Microglia-endothelial cross-talk regulates diabetes-induced retinal vascular dysfunction through remodeling inflammatory microenvironment
- PMID: 38414848
- PMCID: PMC10897849
- DOI: 10.1016/j.isci.2024.109145
Microglia-endothelial cross-talk regulates diabetes-induced retinal vascular dysfunction through remodeling inflammatory microenvironment
Abstract
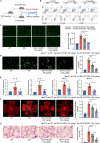
Inflammation-mediated crosstalk between neuroglial cells and endothelial cells (ECs) is a fundamental feature of many vascular diseases. Nevertheless, the landscape of inflammatory processes during diabetes-induced microvascular dysfunction remains elusive. Here, we applied single-cell RNA sequencing to elucidate the transcriptional landscape of diabetic retinopathy (DR). The transcriptome characteristics of microglia and ECs revealed two microglial subpopulations and three EC populations. Exploration of intercellular crosstalk between microglia and ECs showed that diabetes-induced interactions mainly participated in the inflammatory response and vessel development, with colony-stimulating factor 1 (CSF1) and CSF1 receptor (CSF1R) playing important roles in early cell differentiation. Clinically, we found that CSF1/CSF1R crosstalk dysregulation was associated with proliferative DR. Mechanistically, ECs secrete CSF1 and activate CSF1R endocytosis and the CSF1R phosphorylation-mediated MAPK signaling pathway, which elicits the differentiation of microglia and triggers the secretion of inflammatory factors, and subsequently foster angiogenesis by remodeling the inflammatory microenvironment through a positive feedback mechanism.
Keywords: Biological sciences; Diabetology; Endocrinology; Natural sciences.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Triggering Receptor Expressed on Myeloid Cells-2 (TREM2) Interacts With Colony-Stimulating Factor 1 Receptor (CSF1R) but Is Not Necessary for CSF1/CSF1R-Mediated Microglial Survival.Front Immunol. 2021 Mar 25;12:633796. doi: 10.3389/fimmu.2021.633796. eCollection 2021. Front Immunol. 2021. PMID: 33841415 Free PMC article.
-
Rh-CSF1 attenuates neuroinflammation via the CSF1R/PLCG2/PKCε pathway in a rat model of neonatal HIE.J Neuroinflammation. 2020 Jun 10;17(1):182. doi: 10.1186/s12974-020-01862-w. J Neuroinflammation. 2020. PMID: 32522286 Free PMC article.
-
Increased Microglial CSF1R Expression in the SIV/Macaque Model of HIV CNS Disease.J Neuropathol Exp Neurol. 2018 Mar 1;77(3):199-206. doi: 10.1093/jnen/nlx115. J Neuropathol Exp Neurol. 2018. PMID: 29319809 Free PMC article.
-
Neurovascular cross talk in diabetic retinopathy: Pathophysiological roles and therapeutic implications.Am J Physiol Heart Circ Physiol. 2016 Sep 1;311(3):H738-49. doi: 10.1152/ajpheart.00005.2016. Epub 2016 Jul 29. Am J Physiol Heart Circ Physiol. 2016. PMID: 27473938 Free PMC article. Review.
-
Sweet Stress: Coping With Vascular Dysfunction in Diabetic Retinopathy.Front Physiol. 2018 Jul 13;9:820. doi: 10.3389/fphys.2018.00820. eCollection 2018. Front Physiol. 2018. PMID: 30057551 Free PMC article. Review.
Cited by
-
Insights into CSF-1R Expression in the Tumor Microenvironment.Biomedicines. 2024 Oct 18;12(10):2381. doi: 10.3390/biomedicines12102381. Biomedicines. 2024. PMID: 39457693 Free PMC article. Review.
-
New insights of potential biomarkers in diabetic retinopathy: integrated multi-omic analyses.Front Endocrinol (Lausanne). 2025 Jul 1;16:1595207. doi: 10.3389/fendo.2025.1595207. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40666054 Free PMC article. Review.
-
The role of gut microbiota dysbiosis in the inflammatory pathogenesis of diabetic retinopathy.Front Immunol. 2025 Jul 7;16:1604315. doi: 10.3389/fimmu.2025.1604315. eCollection 2025. Front Immunol. 2025. PMID: 40692792 Free PMC article. Review.
-
Application and Progression of Single-Cell RNA Sequencing in Diabetes Mellitus and Diabetes Complications.J Diabetes Res. 2025 Mar 18;2025:3248350. doi: 10.1155/jdr/3248350. eCollection 2025. J Diabetes Res. 2025. PMID: 40135071 Free PMC article. Review.
-
Single-cell RNA sequencing in exploring the pathogenesis of diabetic retinopathy.Clin Transl Med. 2024 Jul;14(7):e1751. doi: 10.1002/ctm2.1751. Clin Transl Med. 2024. PMID: 38946005 Free PMC article. Review.
References
-
- Klemm F., Maas R.R., Bowman R.L., Kornete M., Soukup K., Nassiri S., Brouland J.-P., Iacobuzio-Donahue C.A., Brennan C., Tabar V., et al. Interrogation of the Microenvironmental Landscape in Brain Tumors Reveals Disease-Specific Alterations of Immune Cells. Cell. 2020;181:1643–1660.e17. doi: 10.1016/j.cell.2020.05.007. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

