A Review of New Concepts in Iron Overload
- PMID: 38414914
- PMCID: PMC10895914
A Review of New Concepts in Iron Overload
Abstract
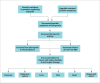
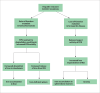
Iron overload disorders are conditions that can lead to increased body iron stores and end-organ damage in affected organs. Increased iron deposition most commonly occurs in the liver, heart, endocrine system, joints, and pancreas. Iron overload disorders may be caused by genetic or acquired causes (transfusion, dyserythropoiesis, and chronic liver disease). The HFE gene C282Y homozygous mutation is the most common cause of hereditary hemochromatosis (HH). Other genes implicated in HH include TFR2, HAMP, HJV, and SLC40A1. In the past 2 decades, there have been major advances in the understanding of genetic iron overload disorders. Furthermore, new novel techniques to measure iron content in organs noninvasively, as well as new therapeutic options for the treatment of HH, are currently under development. This article focuses on the latest concepts in understanding, diagnosing, and managing genetic iron overload disorders, particularly HH.
Keywords: HFE; Iron overload; hemochromatosis; hepcidin mimetics.
Copyright © 2024, Gastro-Hep Communications, Inc.
Figures

Similar articles
-
HFE-Related Hemochromatosis May Be a Primary Kupffer Cell Disease.Biomedicines. 2025 Mar 10;13(3):683. doi: 10.3390/biomedicines13030683. Biomedicines. 2025. PMID: 40149659 Free PMC article. Review.
-
Hereditary hemochromatosis: mutations in genes involved in iron homeostasis in Brazilian patients.Blood Cells Mol Dis. 2011 Apr 15;46(4):302-7. doi: 10.1016/j.bcmd.2011.02.008. Blood Cells Mol Dis. 2011. PMID: 21411349
-
Hepatic iron metabolism gene expression profiles in HFE associated hereditary hemochromatosis.Blood Cells Mol Dis. 2007 Jan-Feb;38(1):37-44. doi: 10.1016/j.bcmd.2006.09.008. Epub 2006 Nov 13. Blood Cells Mol Dis. 2007. PMID: 17098454
-
Identification of Genes for Hereditary Hemochromatosis.Methods Mol Biol. 2018;1706:353-365. doi: 10.1007/978-1-4939-7471-9_19. Methods Mol Biol. 2018. PMID: 29423808 Review.
-
Phenotypic analysis of hemochromatosis subtypes reveals variations in severity of iron overload and clinical disease.Blood. 2018 Jul 5;132(1):101-110. doi: 10.1182/blood-2018-02-830562. Epub 2018 May 9. Blood. 2018. PMID: 29743178
Cited by
-
HFE-Related Hemochromatosis May Be a Primary Kupffer Cell Disease.Biomedicines. 2025 Mar 10;13(3):683. doi: 10.3390/biomedicines13030683. Biomedicines. 2025. PMID: 40149659 Free PMC article. Review.
-
The effects of iron deficient and high iron diets on SARS-CoV-2 lung infection and disease.Front Microbiol. 2024 Sep 4;15:1441495. doi: 10.3389/fmicb.2024.1441495. eCollection 2024. Front Microbiol. 2024. PMID: 39296289 Free PMC article.
-
Studies of Slc30a10 Deficiency in Mice Reveal That Intestinal Iron Transporters Dmt1 and Ferroportin Transport Manganese.Cell Mol Gastroenterol Hepatol. 2025;19(7):101489. doi: 10.1016/j.jcmgh.2025.101489. Epub 2025 Feb 28. Cell Mol Gastroenterol Hepatol. 2025. PMID: 40024532 Free PMC article.
-
Manganese transporter SLC30A10 and iron transporters SLC40A1 and SLC11A2 impact dietary manganese absorption.bioRxiv [Preprint]. 2024 Jul 20:2024.07.17.603814. doi: 10.1101/2024.07.17.603814. bioRxiv. 2024. PMID: 39071439 Free PMC article. Preprint.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
