Retrospective analysis of clinical outcome of 100 inoperable oral cavity carcinoma treated with definitive concurrent chemoradiotherapy with or without induction chemotherapy
- PMID: 38414943
- PMCID: PMC10898909
- DOI: 10.3332/ecancer.2023.1630
Retrospective analysis of clinical outcome of 100 inoperable oral cavity carcinoma treated with definitive concurrent chemoradiotherapy with or without induction chemotherapy
Abstract
Objectives: The management of inoperable oral cavity squamous cell carcinoma (OC-SCC) is onerous. We aimed to retrospectively analyse the outcome of our cohort of inoperable OC-SCC treated with definitive concurrent chemoradiotherapy (CTRT) with or without induction chemotherapy (IC).
Methods: Data of 100 patients (January 2017 to May 2022) of histopathologically proven inoperable OC-SCC treated with definitive CTRT with weekly cisplatin 40 mg/m2 were retrieved from our departmental archives. Radiotherapy (RT) was delivered with three-dimensional conformal plan (66-70 Gy). Toxicities were evaluated using acute morbidity scoring criteria of Radiation Therapy Oncology Group. The response was evaluated as per WHO criteria. Progression free survival (PFS) was calculated from the date of the start of treatment (IC/CTRT) using Kaplan Meier method.
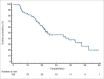
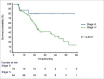
Results: Median age was 45 years (range 30-80 years). The primary site was oral tongue (59%), retro-molar trigon (15%), buccal mucosa (15%) and others (11%). The stage was III: IVA: IVB in 16:70:14 patients respectively. 72% patients received IC (platinum ± 5 FU ± taxane). Grade 3 skin toxicity, oral mucositis and dysphagia was noted in 13 (13%), 19 (19%) and 13 (13%) patients respectively. The median follow-up duration was 30.5 months (range 6-62 months). Complete response (CR), partial response, progressive disease and death at the time of the last follow-up were 49%, 25%, 15% and 11% respectively. 2-year PFS rate was 49.5%. Stage III patients had a higher CR rate (81.2% versus 42.8%; p = 0.0051) and higher 2-year PFS (81.2% versus 46.4%; p = 0.0056) in comparison to stage IV patients.
Conclusion: Inoperable patients of OC-SCC treated with definitive CTRT with or without IC yielded CR in approximately half of patients with acceptable toxicity profiles.
Keywords: carcinoma oral cavity; definitive concurrent chemoradiotherapy; inoperable.
© the authors; licensee ecancermedicalscience.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Final results of a randomized phase III trial of induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in patients with stage IVA and IVB nasopharyngeal carcinoma-Taiwan Cooperative Oncology Group (TCOG) 1303 Study.Ann Oncol. 2018 Sep 1;29(9):1972-1979. doi: 10.1093/annonc/mdy249. Ann Oncol. 2018. PMID: 30016391 Clinical Trial.
-
Neoadjuvant chemotherapy followed by concurrent chemoradiation for locally advanced nasopharyngeal carcinoma.Chin J Cancer. 2010 May;29(5):551-5. doi: 10.5732/cjc.009.10518. Chin J Cancer. 2010. PMID: 20426907 Clinical Trial.
-
Definitive radiotherapy for squamous cell carcinoma of the oral cavity: a single-institution experience.Radiol Oncol. 2021 Nov 19;55(4):467-473. doi: 10.2478/raon-2021-0041. Radiol Oncol. 2021. PMID: 34821134 Free PMC article.
-
Concurrent chemoradiotherapy with vinorelbine plus split-dose cisplatin may be an option in inoperable stage III non-small cell lung cancer: a single-center experience.Med Sci Monit. 2015 Mar 3;21:661-6. doi: 10.12659/MSM.892730. Med Sci Monit. 2015. PMID: 25731741 Free PMC article.
-
Radiotherapy and chemotherapy in locally advanced non-small cell lung cancer: preclinical and early clinical data.Hematol Oncol Clin North Am. 2004 Feb;18(1):41-53. doi: 10.1016/s0889-8588(03)00138-2. Hematol Oncol Clin North Am. 2004. PMID: 15005280 Review.
References
-
- Globocan. 2020. [03/23]. [Internet] [ http://globocan.iarc.fr/Default.aspx]
-
- Hashibe M, Brennan P, Chuang SC, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(2):541–550. doi: 10.1158/1055-9965.EPI-08-0347. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials