Real-world data in patients with BRCA mutated breast cancer treated with poly (ADP-ribose) polymerase inhibitors
- PMID: 38414963
- PMCID: PMC10898914
- DOI: 10.3332/ecancer.2023.1633
Real-world data in patients with BRCA mutated breast cancer treated with poly (ADP-ribose) polymerase inhibitors
Abstract
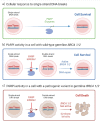
Breast cancer is the most common type of cancer globally. Hereditary breast cancer accounts for 10% of new cases and 4%-5% of cases are associated to pathogenic variants in BRCA1 or BRCA2 genes. In recent years, poly-adenosine-diphosphate-ribose polymerase inhibitors (PARPi) olaparib and talazoparib have been approved for patients with BRCA-associated, HER2 -negative breast cancer. These drugs have shown positive results in the early and advanced setting with a favourable toxicity profile based on the OlympiAD, OlympiA and EMBRACA phase 3 trials. However, patients included in these randomised trials are highly selected, making toxicity and efficacy in patients encountered in routine clinical care a concern. Since the approval of olaparib and talazoparib for advanced human epidermal growth factor receptor 2-negative (HER2-negative) breast cancer, several phase IIIb-IV trials, expanded access cohorts, and retrospective cohorts have provided information on the efficacy and tolerability of these treatments in patient subgroups underrepresented in the registration trials, such as older adults, patients with poor performance status, and heavily pretreated patients. The aim of this review is to present a critical review of the information regarding the use of PARPi in real-world breast cancer patients.
Keywords: BRCA1 gene; BRCA2 gene; breast cancer; poly(ADP-ribose) polymerase inhibitors.
© the authors; licensee ecancermedicalscience.
Conflict of interest statement
Yanin Chávarri-Guerra: Roche research funding and travel expenses; Novartis travel expenses; INVITAE Speakers’ bureau; Astra Zeneca Advisory role. Haydeé Cristina Verduzco-Aguirre: AstraZeneca travel expenses. The rest of the authors declare no conflicts of interest.
Figures
Similar articles
-
LuciA-15 - a real-world prospective study of PARP inhibitors for the treatment of patients with HER-2 negative metastatic breast cancer with germline and/or somatic mutation of BRCA genes or homologous recombination repair related genes.Ecancermedicalscience. 2023 Nov 21;17:1634. doi: 10.3332/ecancer.2023.1634. eCollection 2023. Ecancermedicalscience. 2023. PMID: 38414929 Free PMC article.
-
The role of novel poly (ADP-ribose) inhibitors in the treatment of locally advanced and metastatic Her-2/neu negative breast cancer with inherited germline BRCA1/2 mutations. A review of the literature.J Med Life. 2021 Jan-Mar;14(1):17-20. doi: 10.25122/jml-2020-0132. J Med Life. 2021. PMID: 33767780 Free PMC article. Review.
-
Breast Cancer and Genetic BRCA1/2 Testing in Routine Clinical Practice: Why, When and For Whom?Geburtshilfe Frauenheilkd. 2023 Mar 9;83(3):310-320. doi: 10.1055/a-1929-2629. eCollection 2023 Mar. Geburtshilfe Frauenheilkd. 2023. PMID: 36908286 Free PMC article.
-
Real-world patient-reported outcomes and physician satisfaction with poly (ADP-ribose) polymerase inhibitors versus chemotherapy in patients with germline BRCA1/2-mutated human epidermal growth factor receptor 2-negative advanced breast cancer from the United States, Europe, and Israel.BMC Cancer. 2022 Dec 22;22(1):1343. doi: 10.1186/s12885-022-10325-9. BMC Cancer. 2022. PMID: 36550413 Free PMC article.
-
Efficacy and safety of poly (ADP-ribose) polymerase inhibitors therapy for BRCA-mutated breast cancer: A systematic review and meta-analysis.J Cancer Res Ther. 2021 Dec;17(7):1672-1678. doi: 10.4103/jcrt.jcrt_2085_21. J Cancer Res Ther. 2021. PMID: 35381738
References
-
- Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58 209 women with breast cancer and 101 986 women without the disease. Lancet. 2001;358(9291):1389–1399. doi: 10.1016/S0140-6736(01)06524-2. - DOI - PubMed
-
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
