Real-world safety and effectiveness of secukinumab in adult patients with moderate to severe plaque psoriasis: results from postmarketing surveillance in Korea
- PMID: 38415046
- PMCID: PMC10898308
- DOI: 10.1177/20406223241230180
Real-world safety and effectiveness of secukinumab in adult patients with moderate to severe plaque psoriasis: results from postmarketing surveillance in Korea
Abstract
Background: Secukinumab, a fully human monoclonal antibody, was approved in Korea for the treatment of moderate to severe psoriasis in September 2015.
Objectives: To assess the safety and effectiveness of secukinumab in patients with moderate to severe psoriasis in Korea.
Design: Multicenter, real-world, noninterventional study conducted over 6 years.
Methods: Adults with moderate to severe psoriasis were enrolled. Safety was assessed by evaluating adverse events (AEs), treatment-related AEs, and serious AEs (SAEs). Effectiveness was assessed using the change in absolute Psoriasis Area and Severity Index (PASI) score, percentage of patients achieving PASI 75/90/100 and PASI ⩽2; at weeks 12 and 24.
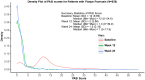
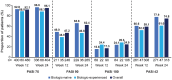
Results: Overall, 829 and 542 patients were included in the safety and effectiveness sets, respectively. AEs, treatment-related AEs, and SAEs occurred in 29.0%, 9.5%, and 4.1% of patients, with incidence rates of 39.43, 12.98, and 5.59 per 100 patient years, respectively. The absolute PASI score decreased from 16.1 ± 7.1 (baseline) to 1.6 ± 2.4 (week 24), with a similar reduction in biologic-naïve (16.4 ± 7.3 to 1.5 ± 2.2) and biologic-experienced (14.8 ± 5.9 to 2.4 ± 3.2) groups. At week 24, PASI 75/90/100 was achieved by 95.1%, 62.4%, and 24.9% of patients. At week 24, PASI 75/90 were higher in biologic-naïve (96.6%/65.8%) than biologic-experienced patients (88.3%/48.6%), whereas PASI 100 was similar in both cohorts (24.1% and 28.6%). A similar trend in PASI ⩽ 2 was observed in both cohorts.
Conclusion: Secukinumab showed sustained effectiveness and favorable safety profile in adult patients with moderate to severe psoriasis in Korea.
Keywords: effectiveness; psoriasis; real-world evidence; safety; secukinumab.
© The Author(s), 2024.
Conflict of interest statement
BSK served as a scientific adviser, a clinical study investigator, and/or a speaker for Abbvie, Astellas, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion, Eli Lilly, Galderma, GlaxoSmithKline, Green Cross, Janssen, Kyowa Hakko Kirin, LEO-Pharma, Novartis, Regeneron, Samsung, Sanofi, and UCB. SJJ reported receiving grants from AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion Healthcare, Green Cross Laboratories, Pfizer, and UCB and personal fees from AbbVie, Eli Lilly and Company, Janssen Pharmaceuticals, LEO Pharma, Novartis, and Sanofi. CHB has served as an investigator in clinical trials sponsored by AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Janssen, Novartis, Pfizer, and UCB biopharma, and has received consultancy fees from AbbVie, Boehringer Ingelheim, Janssen, Kolon Pharma, Lilly, and Novartis, and fees for speaking from AbbVie, Janssen, Lilly, and Novartis. DHK served as a scientific adviser, a clinical study investigator, and/or a speaker for AbbVie, Bristol-Myers Squibb, Celltrion, Eli Lilly, Green Cross, Janssen, LEO-Pharma, Novartis, and UCB. E-SL received grants for clinical research from AbbVie, Inc., Bristol-Myers Squibb, Celgene Corporation, Cell Biotech, Lilly, Janssen Pharmaceuticals, Inc., and Servier; served as an advisor or consultant for AbbVie, Inc., Celgene Corporation, Galderma Korea, Janssen Pharmaceuticals, Inc., Lilly, and Novartis Pharmaceuticals Corporation. YY is an employee of Novartis Korea Ltd. BSS and YBC report no competing interests.
Figures

Similar articles
-
Real-world data from a single Greek centre on the use of secukinumab in plaque psoriasis: effectiveness, safety, drug survival, and identification of patients that sustain optimal response.J Eur Acad Dermatol Venereol. 2020 Jun;34(6):1240-1247. doi: 10.1111/jdv.16202. Epub 2020 Feb 28. J Eur Acad Dermatol Venereol. 2020. PMID: 31953892
-
Effectiveness and safety of secukinumab in Chinese patients with moderate to severe plaque psoriasis in real-world practice.Exp Dermatol. 2024 Jan;33(1):e14890. doi: 10.1111/exd.14890. Epub 2023 Jul 20. Exp Dermatol. 2024. PMID: 37474877
-
Efficacy of Secukinumab Across Subgroups and Overall Safety in Pediatric Patients with Moderate to Severe Plaque Psoriasis: Week 52 Results from a Phase III Randomized Study.Paediatr Drugs. 2022 Jul;24(4):377-387. doi: 10.1007/s40272-022-00507-0. Epub 2022 Jun 13. Paediatr Drugs. 2022. PMID: 35698000 Free PMC article. Clinical Trial.
-
Secukinumab for Treatment of Plaque Psoriasis in Real-World Clinical Practice in Spain: A Literature Review.J Clin Med. 2025 Jan 13;14(2):478. doi: 10.3390/jcm14020478. J Clin Med. 2025. PMID: 39860484 Free PMC article. Review.
-
The efficacy and safety of tyrosine kinase 2 inhibitor deucravacitinib in the treatment of plaque psoriasis: a systematic review and meta-analysis of randomized controlled trials.Front Med (Lausanne). 2023 Sep 29;10:1264667. doi: 10.3389/fmed.2023.1264667. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37841017 Free PMC article.
Cited by
-
Secukinumab Causing Medication-Related Osteonecrosis of the Jaw, in a Patient Diagnosed with Psoriasis and Rheumatoid Arthritis.Psoriasis (Auckl). 2024 Sep 23;14:115-120. doi: 10.2147/PTT.S490982. eCollection 2024. Psoriasis (Auckl). 2024. PMID: 39347517 Free PMC article.
References
-
- Griffiths CEM, Armstrong AW, Gudjonsson JE, et al.. Psoriasis. Lancet 2021; 397: 1301–1315. - PubMed
-
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA 2020; 323: 1945–1960. - PubMed
-
- National Psoriasis Foundation. Psoriasis Statistics, https://www.psoriasis.org/psoriasis-statistics/ (accessed 14 October 2022).
-
- Parisi R, Symmons DP, Griffiths CE, et al.. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013; 133: 377–385. - PubMed
LinkOut - more resources
Full Text Sources

