Identifying content-invariant neural signatures of perceptual vividness
- PMID: 38415219
- PMCID: PMC10898512
- DOI: 10.1093/pnasnexus/pgae061
Identifying content-invariant neural signatures of perceptual vividness
Abstract
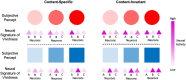
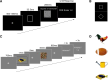
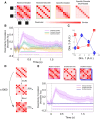
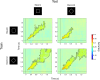
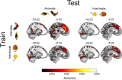
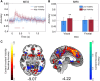
Some conscious experiences are more vivid than others. Although perceptual vividness is a key component of human consciousness, how variation in this magnitude property is registered by the human brain is unknown. A striking feature of neural codes for magnitude in other psychological domains, such as number or reward, is that the magnitude property is represented independently of its sensory features. To test whether perceptual vividness also covaries with neural codes that are invariant to sensory content, we reanalyzed existing magnetoencephalography and functional MRI data from two distinct studies which quantified perceptual vividness via subjective ratings of awareness and visibility. Using representational similarity and decoding analyses, we find evidence for content-invariant neural signatures of perceptual vividness distributed across visual, parietal, and frontal cortices. Our findings indicate that the neural correlates of subjective vividness may share similar properties to magnitude codes in other cognitive domains.
Keywords: MEG; awareness; fMRI; perception.
© The Author(s) 2024. Published by Oxford University Press on behalf of National Academy of Sciences.
Figures
Similar articles
-
Task requirements affect the neural correlates of consciousness.Eur J Neurosci. 2022 Nov;56(10):5810-5822. doi: 10.1111/ejn.15820. Epub 2022 Sep 26. Eur J Neurosci. 2022. PMID: 36086829 Free PMC article.
-
Decoding across sensory modalities reveals common supramodal signatures of conscious perception.Proc Natl Acad Sci U S A. 2020 Mar 31;117(13):7437-7446. doi: 10.1073/pnas.1912584117. Epub 2020 Mar 17. Proc Natl Acad Sci U S A. 2020. PMID: 32184331 Free PMC article.
-
Anchoring visual subjective experience in a neural model: the coarse vividness hypothesis.Neuropsychologia. 2013 May;51(6):1050-60. doi: 10.1016/j.neuropsychologia.2013.02.021. Epub 2013 Mar 13. Neuropsychologia. 2013. PMID: 23499720
-
I Am Conscious, Therefore, I Am: Imagery, Affect, Action, and a General Theory of Behavior.Brain Sci. 2019 May 10;9(5):107. doi: 10.3390/brainsci9050107. Brain Sci. 2019. PMID: 31083483 Free PMC article. Review.
-
The neural correlates of visual imagery vividness - An fMRI study and literature review.Cortex. 2018 Aug;105:26-40. doi: 10.1016/j.cortex.2017.09.014. Epub 2017 Oct 3. Cortex. 2018. PMID: 29079342 Review.
References
-
- Piazza M, Pinel P, Le Bihan D, Dehaene S. 2007. A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron. 53(2):293–305. - PubMed
LinkOut - more resources
Full Text Sources

