Complementary supramolecular drug associates in perfecting the multidrug therapy against multidrug resistant bacteria
- PMID: 38415251
- PMCID: PMC10897028
- DOI: 10.3389/fimmu.2024.1352483
Complementary supramolecular drug associates in perfecting the multidrug therapy against multidrug resistant bacteria
Abstract
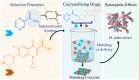
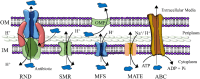
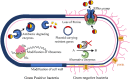
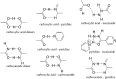
The inappropriate and inconsistent use of antibiotics in combating multidrug-resistant bacteria exacerbates their drug resistance through a few distinct pathways. Firstly, these bacteria can accumulate multiple genes, each conferring resistance to a specific drug, within a single cell. This accumulation usually takes place on resistance plasmids (R). Secondly, multidrug resistance can arise from the heightened expression of genes encoding multidrug efflux pumps, which expel a broad spectrum of drugs from the bacterial cells. Additionally, bacteria can also eliminate or destroy antibiotic molecules by modifying enzymes or cell walls and removing porins. A significant limitation of traditional multidrug therapy lies in its inability to guarantee the simultaneous delivery of various drug molecules to a specific bacterial cell, thereby fostering incremental drug resistance in either of these paths. Consequently, this approach prolongs the treatment duration. Rather than using a biologically unimportant coformer in forming cocrystals, another drug molecule can be selected either for protecting another drug molecule or, can be selected for its complementary activities to kill a bacteria cell synergistically. The development of a multidrug cocrystal not only improves tabletability and plasticity but also enables the simultaneous delivery of multiple drugs to a specific bacterial cell, philosophically perfecting multidrug therapy. By adhering to the fundamental tenets of multidrug therapy, the synergistic effects of these drug molecules can effectively eradicate bacteria, even before they have the chance to develop resistance. This approach has the potential to shorten treatment periods, reduce costs, and mitigate drug resistance. Herein, four hypotheses are presented to create complementary drug cocrystals capable of simultaneously reaching bacterial cells, effectively destroying them before multidrug resistance can develop. The ongoing surge in the development of novel drugs provides another opportunity in the fight against bacteria that are constantly gaining resistance to existing treatments. This endeavour holds the potential to combat a wide array of multidrug-resistant bacteria.
Keywords: binary antibiotic systems; cell membrane permeability; complementary drugs; complementary multidrug cocrystal; crystal engineering; efflux pump; supramolecular synthon.
Copyright © 2024 Sahoo.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Cocrystallizing and Codelivering Complementary Drugs to Multidrugresistant Tuberculosis Bacteria in Perfecting Multidrug Therapy.Curr Top Med Chem. 2023;23(19):1850-1858. doi: 10.2174/1568026623666230504094521. Curr Top Med Chem. 2023. PMID: 37150990
-
Mechanistic Understanding Enables the Rational Design of Salicylanilide Combination Therapies for Gram-Negative Infections.mBio. 2020 Sep 15;11(5):e02068-20. doi: 10.1128/mBio.02068-20. mBio. 2020. PMID: 32934086 Free PMC article.
-
Different Efflux Pump Systems in Acinetobacter baumannii and Their Role in Multidrug Resistance.Adv Exp Med Biol. 2023;1370:155-168. doi: 10.1007/5584_2023_771. Adv Exp Med Biol. 2023. PMID: 36971967 Review.
-
Emergence of a Potent Multidrug Efflux Pump Variant That Enhances Campylobacter Resistance to Multiple Antibiotics.mBio. 2016 Sep 20;7(5):e01543-16. doi: 10.1128/mBio.01543-16. mBio. 2016. PMID: 27651364 Free PMC article.
-
Inhibitors of multidrug resistant efflux systems in bacteria.Recent Pat Antiinfect Drug Discov. 2009 Jan;4(1):37-50. doi: 10.2174/157489109787236256. Recent Pat Antiinfect Drug Discov. 2009. PMID: 19149695 Review.
Cited by
-
Protein-based nanoparticles for antimicrobial and cancer therapy: implications for public health.RSC Adv. 2025 May 8;15(19):14966-15016. doi: 10.1039/d5ra01427a. eCollection 2025 May 6. RSC Adv. 2025. PMID: 40343307 Free PMC article. Review.
-
Phytochemical Composition and Bioactivities of Some Hydrophytes: Antioxidant, Antiparasitic, Antibacterial, and Anticancer Properties and Mechanisms.Plants (Basel). 2024 Aug 2;13(15):2148. doi: 10.3390/plants13152148. Plants (Basel). 2024. PMID: 39124266 Free PMC article.
-
Self-Healing Hydrogels with Intrinsic Antioxidant and Antibacterial Properties Based on Oxidized Hydroxybutanoyl Glycan and Quaternized Carboxymethyl Chitosan for pH-Responsive Drug Delivery.Gels. 2025 Feb 26;11(3):169. doi: 10.3390/gels11030169. Gels. 2025. PMID: 40136873 Free PMC article.
-
Avibactam-Cyclodextrin Inclusion Complexes: Computational and Thermodynamic Insights for Drug Delivery, Detection, and Environmental Scavenging.Molecules. 2025 Aug 18;30(16):3401. doi: 10.3390/molecules30163401. Molecules. 2025. PMID: 40871555 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

