Secretion of Sphinganine by Drug-Induced Cancer Cells and Modified Mimetic Sphinganine (MMS) as c-Src Kinase Inhibitor
- PMID: 38415528
- PMCID: PMC11077104
- DOI: 10.31557/APJCP.2024.25.2.433
Secretion of Sphinganine by Drug-Induced Cancer Cells and Modified Mimetic Sphinganine (MMS) as c-Src Kinase Inhibitor
Abstract
Background: Cancer cells exhibit selective metabolic reprogramming to promote proliferation, invasiveness, and metastasis. Sphingolipids such as sphingosine and sphinganine have been reported to modulate cell death processes in cancer cells. However, the potential of extracellular sphinganine and its mimetic compounds as inducers of cancer cell death has not been thoroughly investigated.
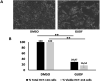
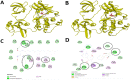
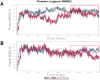
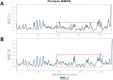
Methods: We obtained extracellular conditioned medium from HCT-116 cells treated with the previously reported anticancer composition, goat urine DMSO fraction (GUDF). The extracellular metabolites were purified using a novel and in-house developed vertical tube gel electrophoresis (VTGE) technique and identified through LC-HRMS. Extracellular metabolites such as sphinganine, sphingosine, C16 sphinganine, and phytosphingosine were screened for their inhibitory role against intracellular kinases using molecular docking. Molecular dynamics (MD) simulations were performed to study the inhibitory potential of a novel designed modified mimetic sphinganine (MMS) (Pubchem CID: 162625115) upon c-Src kinase. Furthermore, inhibitory potential and ADME profile of MMS was compared with luteolin, a known c-Src kinase inhibitor.
Results: Data showed accumulation of sphinganine and other sphingolipids such as C16 sphinganine, phytosphingosine, and ceramide (d18:1/14:0) in the extracellular compartment of GUDF-treated HCT-116 cells. Molecular docking projected c-Src kinase as an inhibitory target of sphinganine. MD simulations projected MMS with strong (-7.1 kcal/mol) and specific (MET341, ASP404) binding to the inhibitory pocket of c-Src kinase. The projected MMS showed comparable inhibitory role and acceptable ADME profile over known inhibitors.
Conclusion: In summary, our findings highlight the significance of extracellular sphinganine and other sphingolipids, including C16 sphinganine, phytosphingosine, and ceramide (d18:1/14:0), in the context of drug-induced cell death in HCT-116 cancer cells. Furthermore, we demonstrated the importance of extracellular sphinganine and its modified mimetic sphinganine (MMS) as a potential inhibitor of c-Src kinase. These findings suggest that MMS holds promise for future applications in targeted and combinatorial anticancer therapy.
Keywords: HCT-116; neoplasm; therapy.
Conflict of interest statement
The authors declare no conflict of interests.
Figures





Similar articles
-
Intracellular Ellagic Acid Derived from Goat Urine DMSO Fraction (GUDF) Predicted as an Inhibitor of c-Raf Kinase.Curr Mol Med. 2024;24(2):264-279. doi: 10.2174/1566524023666230113141032. Curr Mol Med. 2024. PMID: 36642883
-
Sphingoid bases and ceramide induce apoptosis in HT-29 and HCT-116 human colon cancer cells.Exp Biol Med (Maywood). 2002 May;227(5):345-53. doi: 10.1177/153537020222700507. Exp Biol Med (Maywood). 2002. PMID: 11976405
-
Differential roles of de novo sphingolipid biosynthesis and turnover in the "burst" of free sphingosine and sphinganine, and their 1-phosphates and N-acyl-derivatives, that occurs upon changing the medium of cells in culture.J Biol Chem. 1995 Aug 11;270(32):18749-58. doi: 10.1074/jbc.270.32.18749. J Biol Chem. 1995. PMID: 7642524
-
Sphingolipid perturbations as mechanisms for fumonisin carcinogenesis.Environ Health Perspect. 2001 May;109 Suppl 2(Suppl 2):301-8. doi: 10.1289/ehp.01109s2301. Environ Health Perspect. 2001. PMID: 11359699 Free PMC article. Review.
-
Activation of sphingosine kinase-1 in cancer: implications for therapeutic targeting.Curr Mol Pharmacol. 2010 Jun;3(2):53-65. doi: 10.2174/1874467211003020053. Curr Mol Pharmacol. 2010. PMID: 20302564 Review.
Cited by
-
Recent Progress in the Diverse Synthetic Approaches to Phytosphingosine.Top Curr Chem (Cham). 2025 Aug 31;383(3):35. doi: 10.1007/s41061-025-00518-8. Top Curr Chem (Cham). 2025. PMID: 40886224 Review.
-
Challenges in Metabolite Biomarkers as Avenues of Diagnosis and Prognosis of Cancer.J Cancer Prev. 2024 Dec 30;29(4):105-112. doi: 10.15430/JCP.24.015. J Cancer Prev. 2024. PMID: 39790219 Free PMC article.
-
Expanding horizons of cancer immunotherapy: hopes and hurdles.Front Oncol. 2025 Apr 25;15:1511560. doi: 10.3389/fonc.2025.1511560. eCollection 2025. Front Oncol. 2025. PMID: 40352591 Free PMC article. Review.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–74. - PubMed
-
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–64. - PubMed
-
- Kawamori T, Osta W, Johnson KR, Pettus BJ, Bielawski J, Tanaka T, et al. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. Faseb J. 2006;20(2):386–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

