Genetic Basis and Evolution of Structural Color Polymorphism in an Australian Songbird
- PMID: 38415852
- PMCID: PMC10962638
- DOI: 10.1093/molbev/msae046
Genetic Basis and Evolution of Structural Color Polymorphism in an Australian Songbird
Abstract
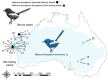
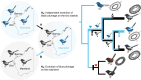
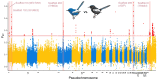
Island organisms often evolve phenotypes divergent from their mainland counterparts, providing a useful system for studying adaptation under differential selection. In the white-winged fairywren (Malurus leucopterus), subspecies on two islands have a black nuptial plumage whereas the subspecies on the Australian mainland has a blue nuptial plumage. The black subspecies have a feather nanostructure that could in principle produce a blue structural color, suggesting a blue ancestor. An earlier study proposed independent evolution of melanism on the islands based on the history of subspecies divergence. However, the genetic basis of melanism and the origin of color differentiation in this group are still unknown. Here, we used whole-genome resequencing to investigate the genetic basis of melanism by comparing the blue and black M. leucopterus subspecies to identify highly divergent genomic regions. We identified a well-known pigmentation gene ASIP and four candidate genes that may contribute to feather nanostructure development. Contrary to the prediction of convergent evolution of island melanism, we detected signatures of a selective sweep in genomic regions containing ASIP and SCUBE2 not in the black subspecies but in the blue subspecies, which possesses many derived SNPs in these regions, suggesting that the mainland subspecies has re-evolved a blue plumage from a black ancestor. This proposed re-evolution was likely driven by a preexisting female preference. Our findings provide new insight into the evolution of plumage coloration in island versus continental populations, and, importantly, we identify candidate genes that likely play roles in the development and evolution of feather structural coloration.
Keywords: avian plumage color; color genetics; feather coloration; male ornamentation; structural color; white-winged fairywren.
© The Author(s) 2024. Published by Oxford University Press on behalf of Society for Molecular Biology and Evolution.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Patterns of genetic divergence and demographic history shed light on island-mainland population dynamics and melanic plumage evolution in the white-winged Fairywren.Evolution. 2021 Jun;75(6):1348-1360. doi: 10.1111/evo.14185. Epub 2021 Feb 21. Evolution. 2021. PMID: 33543771
-
Concordant evolution of plumage colour, feather microstructure and a melanocortin receptor gene between mainland and island populations of a fairy-wren.Proc Biol Sci. 2004 Aug 22;271(1549):1663-70. doi: 10.1098/rspb.2004.2779. Proc Biol Sci. 2004. PMID: 15306285 Free PMC article.
-
Selective sweeps on different pigmentation genes mediate convergent evolution of island melanism in two incipient bird species.PLoS Genet. 2022 Nov 1;18(11):e1010474. doi: 10.1371/journal.pgen.1010474. eCollection 2022 Nov. PLoS Genet. 2022. PMID: 36318577 Free PMC article.
-
Avian Coloration Genetics: Recent Advances and Emerging Questions.J Hered. 2021 Aug 25;112(5):395-416. doi: 10.1093/jhered/esab015. J Hered. 2021. PMID: 34002228 Review.
-
Diversity, physiology, and evolution of avian plumage carotenoids and the role of carotenoid-protein interactions in plumage color appearance.Arch Biochem Biophys. 2015 Apr 15;572:201-212. doi: 10.1016/j.abb.2015.01.016. Epub 2015 Jan 28. Arch Biochem Biophys. 2015. PMID: 25637658 Review.
References
-
- Bonser RH. Melanin and the abrasion resistance of feathers. Condor. 1995:97(2):590–591. 10.2307/1369048. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources

