Sirtuin2 suppresses the polarization of regulatory T cells toward T helper 17 cells through repressing the expression of signal transducer and activator of transcription 3 in a mouse colitis model
- PMID: 38415949
- PMCID: PMC10836035
- DOI: 10.1002/iid3.1160
Sirtuin2 suppresses the polarization of regulatory T cells toward T helper 17 cells through repressing the expression of signal transducer and activator of transcription 3 in a mouse colitis model
Abstract
Introduction: Regulatory T cells (Tregs) play an important role in inflammatory bowel diseases (IBDs) through modulating intestinal inflammation. However, the factors affecting Treg function and plasticity during IBD progression are not thoroughly disclosed. The current study aims to reveal new molecular mechanisms affecting Treg plasticity.
Methods: A mouse strain, in which tdTomato and enhanced green fluorescent protein were under the control of the Foxp3 promoter and Il17a promoter, was established and subjected to colitis induction with dextran sulfate sodium. The existence of Tregs and IL-17-expressing Tregs (i.e., Treg/T helper 17 [Th17] cells) were observed and sorted from the spleen, mesenteric lymph nodes, and lamina propria by flow cytometry, followed by measuring Sirtuin2 (Sirt2) expression using quantitative reverse transcription polymerase chain reaction and Immunoblotting. Lentivirus-induced Sirt2 silencing was applied to determine the impact of Sirt2 on Treg polarization to Treg/Th17 cells and even Th17 cells. The effect of Sirt2 on Stat3 was analyzed by flow cytometry and immunoblotting.
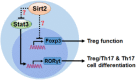
Results: Sirt2 was highly expressed in lamina propria Tregs and it moderately suppressed Foxp3 expression as well as the immunosuppressive function of Tregs. Surprisingly, lentivirus-mediated Sirt2 silencing promoted the generation of Treg/Th17 cells out of Tregs. Sirt2 silencing also enhanced the generation of Th17 cells out of Tregs under the Th17 induction condition. Furthermore, Sirt2 inhibited Th17 induction by suppressing the protein level of the signal transducer and activator of transcription 3.
Conclusion: Sirt2 suppresses Treg function but also inhibits Treg polarization toward Treg/Th17 cells and Th17 cells. The ultimate effect of Sirt2 on colitis might depend on the balance among Tregs, Treg/Th17 cells, and Th17 cells.
Keywords: Sirtuin2; T helper 17 cells; inflammatory bowel disease; regulatory T cells; signal transducer and activator of transcription 3.
© 2024 The Authors. Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
The canonical Hippo pathway components modulate the differentiation of lamina propria regulatory T cells and T helper 17-like regulatory T cells in mouse colitis.Int Immunol. 2024 Nov 25;37(1):25-38. doi: 10.1093/intimm/dxae043. Int Immunol. 2024. PMID: 38955508
-
Thiomyristoyl ameliorates colitis by blocking the differentiation of Th17 cells and inhibiting SIRT2-induced metabolic reprogramming.Int Immunopharmacol. 2021 Jan;90:107212. doi: 10.1016/j.intimp.2020.107212. Epub 2020 Dec 11. Int Immunopharmacol. 2021. PMID: 33310666
-
Heme oxygenase-1 ameliorates dextran sulfate sodium-induced acute murine colitis by regulating Th17/Treg cell balance.J Biol Chem. 2014 Sep 26;289(39):26847-26858. doi: 10.1074/jbc.M114.590554. Epub 2014 Aug 11. J Biol Chem. 2014. PMID: 25112868 Free PMC article.
-
SIRT3 Activator Honokiol Inhibits Th17 Cell Differentiation and Alleviates Colitis.Inflamm Bowel Dis. 2023 Dec 5;29(12):1929-1940. doi: 10.1093/ibd/izad099. Inflamm Bowel Dis. 2023. PMID: 37335900 Free PMC article.
-
T helper cell 17/regulatory T cell balance regulates ulcerative colitis and the therapeutic role of natural plant components: a review.Front Med (Lausanne). 2025 Mar 24;11:1502849. doi: 10.3389/fmed.2024.1502849. eCollection 2024. Front Med (Lausanne). 2025. PMID: 40196424 Free PMC article. Review.
Cited by
-
Challenges in the preclinical design and assessment of CAR-T cells.Front Immunol. 2025 Aug 8;16:1564998. doi: 10.3389/fimmu.2025.1564998. eCollection 2025. Front Immunol. 2025. PMID: 40861448 Free PMC article. Review.
References
-
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521‐533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

