Effects of myeloperoxidase on inflammatory responses with hypoxia in Citrobacter rodentium-infectious mice
- PMID: 38415976
- PMCID: PMC10836036
- DOI: 10.1002/iid3.1157
Effects of myeloperoxidase on inflammatory responses with hypoxia in Citrobacter rodentium-infectious mice
Abstract
Purpose: Myeloperoxidase (MPO) has been identified as a mediator in various inflammatory diseases. Bacterial infection of the intestine and hypoxia can both lead to inflammatory responses, but the role of MPO in these phenomena remains unclear.
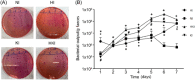
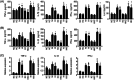
Methods: By building the MPO-/- mice, we evaluated relevant inflammatory factors and tissue damage in mice with intestinal Citrobacter rodentium infection and hypoxia. The body weight and excreted microorganisms were monitored. Intestinal tissues were collected 7 days after bacterial infection under hypoxia to undergo haematoxylin-eosin staining and assess the degree of pathological damage. ELISA assays were performed to quantify the serum levels of TNF-α, IFN-γ, IL-6, and IL-1β inflammatory cytokines. PCR, WB, and IF assays were conducted to determine the expression of chemokines MCP1, MIP2, and KC in the colon and spleen.
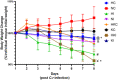
Results: The C. rodentium infection and hypoxia caused weight loss, intestinal colitis, and splenic inflammatory cells active proliferation in wild-type mice. MPO deficiency alleviated this phenomenon. MPO-/- mice also displayed a significant decline in bacteria clearing ability. The level of TNF-α in the serum and spleen was both lower in MPO-/- hypoxia C. rodentium-infected mice than that in wild-type mice. The chemokines expression levels of MIP2, KC, and MCP1 in the spleen and colon of each bacterial infected group were significantly increased (p < .05), while in hypoxia, the factors in the spleen and colon were decreased (p < .05). MPO deficiency was found to lower the levels of these chemokines compared with wild-type mice.
Conclusion: MPO plays an important role of the inflammatory responses in infectious enteritis and hypoxia in mice, and the loss of MPO may greatly reduce the body's inflammatory responses to fight diseases.
Keywords: MPO; colitis; hypoxia; inflammatory responses.
© 2024 The Authors. Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Comment on
-
Myeloperoxidase in the inflamed colon: A novel target for treating inflammatory bowel disease.Arch Biochem Biophys. 2018 May 1;645:61-71. doi: 10.1016/j.abb.2018.03.012. Epub 2018 Mar 13. Arch Biochem Biophys. 2018. PMID: 29548776 Review.
References
-
- O'Brien PJ. Peroxidases. Chem Biol Interact. 2000;129(1‐2):113‐139. - PubMed
-
- Nakabo S, Ohmura K, Akizuki S, et al. Activated neutrophil carbamylates albumin via the release of myeloperoxidase and reactive oxygen species regardless of NETosis. Modern Rheumatol. 2020;30(2):345‐349. - PubMed
-
- Galijasevic S. The development of myeloperoxidase inhibitors. Bioorg Med Chem Lett. 2019;29(1):1‐7. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

