Effect of Spironolactone on Kidney Function in Kidney Transplant Recipients (the SPIREN trial): A Randomized Placebo-Controlled Clinical Trial
- PMID: 38416033
- PMCID: PMC11168825
- DOI: 10.2215/CJN.0000000000000439
Effect of Spironolactone on Kidney Function in Kidney Transplant Recipients (the SPIREN trial): A Randomized Placebo-Controlled Clinical Trial
Abstract
Key Points:
Spironolactone is safe for kidney transplant patients.
Spironolactone reduces kidney function by an acute effect, whereafter it remains stable.
Spironolactone does not affect the progression of interstitial fibrosis in protocol biopsies.
Background: Long-term kidney allograft survival is hampered by progressive interstitial fibrosis and tubular atrophy. The SPIREN trial tested the hypothesis that the mineralocorticoid receptor antagonist spironolactone stabilizes kidney function and attenuates glomerular barrier injury in kidney transplant patients treated with calcineurin inhibitors.
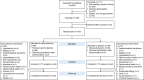
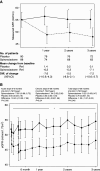
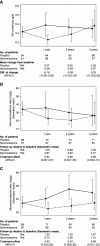
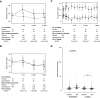
Methods: We conducted a randomized, placebo-controlled, double-blind clinical trial including 188 prevalent kidney transplant patients. Patients were randomized to spironolactone or placebo for 3 years. GFR was measured along with proteinuria and kidney fibrosis. The primary end point was change in measured GFR. Secondary outcomes were 24-hour proteinuria, kidney allograft fibrosis, and cardiovascular events. Measured GFRs, 24-hour proteinuria, and BP were determined yearly. Kidney biopsies were collected at baseline and after 2 years (n=48). Fibrosis was evaluated by quantitative stereology and classified according to Banff.
Results: The groups were comparable at baseline except for slightly older allografts in the spironolactone group. Spironolactone reduced measured GFRs (up to –7.6 [95% confidence interval, −10.9 to −4.3] ml/min compared with placebo) independently of time since transplantation and BP with no effect on the kidney function curve over time and reduced 24-hour proteinuria after 1 year. There was no significant effect of spironolactone on the development of interstitial fibrosis.
Conclusions: Spironolactone added to standard therapy for 3 years in kidney transplant patients did not improve kidney function, long-term proteinuria, or interstitial fibrosis.
Clinical Trial registration number:
Conflict of interest statement
L. Boesby received personal honoraria from Astellas and AstraZeneca and participated in an advisory board for Vifor Pharma—none of these relating to the present study. N. Marcussen reports Consultancy: Novo Nordisk A/S. L.A. Mortensen is the principal investigator of the FIND-CKD study (
Figures


Similar articles
-
The effect of spironolactone on calcineurin inhibitor induced nephrotoxicity: a multicenter randomized, double-blind, clinical trial (the SPIREN trial).BMC Nephrol. 2018 May 3;19(1):105. doi: 10.1186/s12882-018-0885-6. BMC Nephrol. 2018. PMID: 29724188 Free PMC article. Clinical Trial.
-
Spironolactone reduces oxidative stress in living donor kidney transplantation: a randomized controlled trial.Am J Physiol Renal Physiol. 2019 Sep 1;317(3):F519-F528. doi: 10.1152/ajprenal.00606.2018. Epub 2019 Jun 26. Am J Physiol Renal Physiol. 2019. PMID: 31241992 Clinical Trial.
-
Effect of spironolactone for 1 yr on endothelial function and vascular inflammation biomarkers in renal transplant recipients.Am J Physiol Renal Physiol. 2019 Sep 1;317(3):F529-F539. doi: 10.1152/ajprenal.00025.2019. Epub 2019 Jun 5. Am J Physiol Renal Physiol. 2019. PMID: 31166706 Clinical Trial.
-
Aldosterone antagonists in addition to renin angiotensin system antagonists for preventing the progression of chronic kidney disease.Cochrane Database Syst Rev. 2020 Oct 27;10(10):CD007004. doi: 10.1002/14651858.CD007004.pub4. Cochrane Database Syst Rev. 2020. PMID: 33107592 Free PMC article.
-
Spironolactone Add-on for Preventing or Slowing the Progression of Diabetic Nephropathy: A Meta-analysis.Clin Ther. 2015 Sep;37(9):2086-2103.e10. doi: 10.1016/j.clinthera.2015.05.508. Epub 2015 Aug 5. Clin Ther. 2015. PMID: 26254276 Review.
Cited by
-
Mineralocorticoid receptor antagonism for non-diabetic kidney disease.Nephrol Dial Transplant. 2025 Feb 5;40(Supplement_1):i29-i36. doi: 10.1093/ndt/gfae241. Nephrol Dial Transplant. 2025. PMID: 39907538 Free PMC article. Review.
-
Recent Advances and Perspectives on the Use of Mineralocorticoid Receptor Antagonists for the Treatment of Hypertension and Chronic Kidney Disease: A Review.Biomedicines. 2024 Dec 29;13(1):53. doi: 10.3390/biomedicines13010053. Biomedicines. 2024. PMID: 39857638 Free PMC article. Review.
-
Calcineurin Inhibitor Associated Nephrotoxicity in Kidney Transplantation-A Transplant Nephrologist's Perspective.Acta Physiol (Oxf). 2025 May;241(5):e70047. doi: 10.1111/apha.70047. Acta Physiol (Oxf). 2025. PMID: 40243357 Free PMC article. Review.
-
Benefit of Renin Angiotensin Aldosterone Blockade in Kidney Transplant Recipients: Is the Verdict in or Still to Be Answered?Clin J Am Soc Nephrol. 2024 Jun 1;19(6):691-693. doi: 10.2215/CJN.0000000000000479. Epub 2024 May 17. Clin J Am Soc Nephrol. 2024. PMID: 38758607 Free PMC article. No abstract available.
-
Mineralocorticoid receptor blockage in kidney transplantation: too much of a good thing or not?Int Urol Nephrol. 2025 Mar;57(3):839-854. doi: 10.1007/s11255-024-04256-6. Epub 2024 Oct 29. Int Urol Nephrol. 2025. PMID: 39470940 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

