Plitidepsin as an Immunomodulator against Respiratory Viral Infections
- PMID: 38416036
- PMCID: PMC10984758
- DOI: 10.4049/jimmunol.2300426
Plitidepsin as an Immunomodulator against Respiratory Viral Infections
Abstract
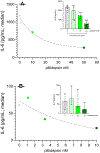
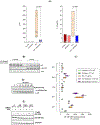
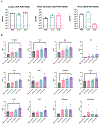
Plitidepsin is a host-targeted compound known for inducing a strong anti-SARS-CoV-2 activity, as well as for having the capacity of reducing lung inflammation. Because IL-6 is one of the main cytokines involved in acute respiratory distress syndrome, the effect of plitidepsin in IL-6 secretion in different in vitro and in vivo experimental models was studied. A strong plitidepsin-mediated reduction of IL-6 was found in human monocyte-derived macrophages exposed to nonproductive SARS-CoV-2. In resiquimod (a ligand of TLR7/8)-stimulated THP1 human monocytes, plitidepsin-mediated reductions of IL-6 mRNA and IL-6 levels were also noticed. Additionally, although resiquimod-induced binding to DNA of NF-κB family members was unaffected by plitidepsin, a decrease in the regulated transcription by NF-κB (a key transcription factor involved in the inflammatory cascade) was observed. Furthermore, the phosphorylation of p65 that is required for full transcriptional NF-κB activity was significantly reduced by plitidepsin. Moreover, decreases of IL-6 levels and other proinflammatory cytokines were also seen in either SARS-CoV-2 or H1N1 influenza virus-infected mice, which were treated at low enough plitidepsin doses to not induce antiviral effects. In summary, plitidepsin is a promising therapeutic agent for the treatment of viral infections, not only because of its host-targeted antiviral effect, but also for its immunomodulatory effect, both of which were evidenced in vitro and in vivo by the decrease of proinflammatory cytokines.
Copyright © 2024 by The American Association of Immunologists, Inc.
Figures


Similar articles
-
Phillyrin (KD-1) exerts anti-viral and anti-inflammatory activities against novel coronavirus (SARS-CoV-2) and human coronavirus 229E (HCoV-229E) by suppressing the nuclear factor kappa B (NF-κB) signaling pathway.Phytomedicine. 2020 Nov;78:153296. doi: 10.1016/j.phymed.2020.153296. Epub 2020 Aug 1. Phytomedicine. 2020. PMID: 32890913 Free PMC article.
-
Antiviral effect of fufang yinhua jiedu (FFYH) granules against influenza A virus through regulating the inflammatory responses by TLR7/MyD88 signaling pathway.J Ethnopharmacol. 2021 Jul 15;275:114063. doi: 10.1016/j.jep.2021.114063. Epub 2021 Apr 1. J Ethnopharmacol. 2021. PMID: 33813013 Free PMC article.
-
Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A.Science. 2021 Feb 26;371(6532):926-931. doi: 10.1126/science.abf4058. Epub 2021 Jan 25. Science. 2021. PMID: 33495306 Free PMC article.
-
[Plitidepsin, an inhibitor of the cell elongation factor eEF1a, and molnupiravir an analogue of the ribonucleoside cytidine, two new chemical compounds with intense activity against SARS-CoV-2].Rev Esp Quimioter. 2021 Oct;34(5):402-407. doi: 10.37201/req/042.2021. Epub 2021 Apr 27. Rev Esp Quimioter. 2021. PMID: 33902254 Free PMC article. Review. Spanish.
-
Plitidepsin: Mechanisms and Clinical Profile of a Promising Antiviral Agent against COVID-19.J Pers Med. 2021 Jul 16;11(7):668. doi: 10.3390/jpm11070668. J Pers Med. 2021. PMID: 34357135 Free PMC article. Review.
References
-
- Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, O’Meara MJ, Rezelj VV, Guo JZ, Swaney DL, Tummino TA, Huttenhain R, Kaake RM, Richards AL, Tutuncuoglu B, Foussard H, Batra J, Haas K, Modak M, Kim M, Haas P, Polacco BJ, Braberg H, Fabius JM, Eckhardt M, Soucheray M, Bennett MJ, Cakir M, McGregor MJ, Li Q, Meyer B, Roesch F, Vallet T, Mac Kain A, Miorin L, Moreno E, Naing ZZC, Zhou Y, Peng S, Shi Y, Zhang Z, Shen W, Kirby IT, Melnyk JE, Chorba JS, Lou K, Dai SA, Barrio-Hernandez I, Memon D, Hernandez-Armenta C, Lyu J, Mathy CJP, Perica T, Pilla KB, Ganesan SJ, Saltzberg DJ, Rakesh R, Liu X, Rosenthal SB, Calviello L, Venkataramanan S, Liboy-Lugo J, Lin Y, Huang XP, Liu Y, Wankowicz SA, Bohn M, Safari M, Ugur FS, Koh C, Savar NS, Tran QD, Shengjuler D, Fletcher SJ, O’Neal MC, Cai Y, Chang JCJ, Broadhurst DJ, Klippsten S, Sharp PP, Wenzell NA, Kuzuoglu-Ozturk D, Wang HY, Trenker R, Young JM, Cavero DA, Hiatt J, Roth TL, Rathore U, Subramanian A, Noack J, Hubert M, Stroud RM, Frankel AD, Rosenberg OS, Verba KA, Agard DA, Ott M, Emerman M, Jura N, von Zastrow M, Verdin E, Ashworth A, Schwartz O, d’Enfert C, Mukherjee S, Jacobson M, Malik HS, Fujimori DG, Ideker T, Craik CS, Floor SN, Fraser JS, Gross JD, Sali A, Roth BL, Ruggero D, Taunton J, Kortemme T, Beltrao P, Vignuzzi M, Garcia-Sastre A, Shokat KM, Shoichet BK, and Krogan NJ. 2020. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583: 459–468. - PMC - PubMed
-
- Rodon J, Muñoz-Basagoiti J, Perez-Zsolt D, Noguera-Julian M, Paredes R, Mateu L, Quiñones C, Perez C, Erkizia I, Blanco I, Valencia A, Guallar V, Carrillo J, Blanco J, Segalés J, Clotet B, Vergara-Alert J, and Izquierdo-Useros N. 2021. Identification of Plitidepsin as Potent Inhibitor of SARS-CoV-2-Induced Cytopathic Effect After a Drug Repurposing Screen. Frontiers in Pharmacology 12. - PMC - PubMed
-
- White KM, Rosales R, Yildiz S, Kehrer T, Miorin L, Moreno E, Jangra S, Uccellini MB, Rathnasinghe R, Coughlan L, Martinez-Romero C, Batra J, Rojc A, Bouhaddou M, Fabius JM, Obernier K, Dejosez M, Guillen MJ, Losada A, Aviles P, Schotsaert M, Zwaka T, Vignuzzi M, Shokat KM, Krogan NJ, and Garcia-Sastre A. 2021. Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science 371: 926–931. - PMC - PubMed
-
- Sachse M, Tenorio R, Fernandez de Castro I, Munoz-Basagoiti J, Perez-Zsolt D, Raich-Regue D, Rodon J, Losada A, Aviles P, Cuevas C, Paredes R, Segales J, Clotet B, Vergara-Alert J, Izquierdo-Useros N, and Risco C. 2022. Unraveling the antiviral activity of plitidepsin against SARS-CoV-2 by subcellular and morphological analysis. Antiviral Res 200: 105270. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

