Deep-Learning Model for Tumor-Type Prediction Using Targeted Clinical Genomic Sequencing Data
- PMID: 38416134
- PMCID: PMC11145170
- DOI: 10.1158/2159-8290.CD-23-0996
Deep-Learning Model for Tumor-Type Prediction Using Targeted Clinical Genomic Sequencing Data
Abstract
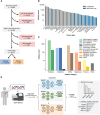
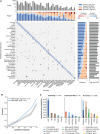
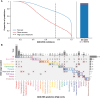
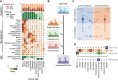
Tumor type guides clinical treatment decisions in cancer, but histology-based diagnosis remains challenging. Genomic alterations are highly diagnostic of tumor type, and tumor-type classifiers trained on genomic features have been explored, but the most accurate methods are not clinically feasible, relying on features derived from whole-genome sequencing (WGS), or predicting across limited cancer types. We use genomic features from a data set of 39,787 solid tumors sequenced using a clinically targeted cancer gene panel to develop Genome-Derived-Diagnosis Ensemble (GDD-ENS): a hyperparameter ensemble for classifying tumor type using deep neural networks. GDD-ENS achieves 93% accuracy for high-confidence predictions across 38 cancer types, rivaling the performance of WGS-based methods. GDD-ENS can also guide diagnoses of rare type and cancers of unknown primary and incorporate patient-specific clinical information for improved predictions. Overall, integrating GDD-ENS into prospective clinical sequencing workflows could provide clinically relevant tumor-type predictions to guide treatment decisions in real time.
Significance: We describe a highly accurate tumor-type prediction model, designed specifically for clinical implementation. Our model relies only on widely used cancer gene panel sequencing data, predicts across 38 distinct cancer types, and supports integration of patient-specific nongenomic information for enhanced decision support in challenging diagnostic situations. See related commentary by Garg, p. 906. This article is featured in Selected Articles from This Issue, p. 897.
©2024 The Authors; Published by the American Association for Cancer Research.
Figures
Update of
-
Deep Learning Model for Tumor Type Prediction using Targeted Clinical Genomic Sequencing Data.medRxiv [Preprint]. 2023 Sep 10:2023.09.08.23295131. doi: 10.1101/2023.09.08.23295131. medRxiv. 2023. Update in: Cancer Discov. 2024 Jun 3;14(6):1064-1081. doi: 10.1158/2159-8290.CD-23-0996. PMID: 37732244 Free PMC article. Updated. Preprint.
References
-
- Pavlidis N, Briasoulis E, Hainsworth J, Greco FA. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer 2003;39:1990–2005. - PubMed
-
- Greco FA. Molecular diagnosis of the tissue of origin in cancer of unknown primary site: useful in patient management. Curr Treat Options Oncol 2013;14:634–42. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

