Durvalumab Plus Gemcitabine and Cisplatin in Patients with Advanced Biliary Tract Cancer: An Exploratory Analysis of Real-World Data
- PMID: 38416377
- PMCID: PMC10963573
- DOI: 10.1007/s11523-024-01044-1
Durvalumab Plus Gemcitabine and Cisplatin in Patients with Advanced Biliary Tract Cancer: An Exploratory Analysis of Real-World Data
Abstract
Background: The combination of gemcitabine and cisplatin (gem/cis) with the anti-PD-L1-antibody durvalumab was recently approved as first line therapy for biliary tract cancer (BTC) based on the results of the TOPAZ-1 trial.
Objective: We aim to analyse the feasibility and efficacy of the triple combination therapy in patients with BTC in a real-world setting and in correspondence with the genetic alterations of the cancer.
Methods: In this single-centre retrospective analysis, all patients with BTC and treated with durvalumab plus gem/cis from April 2022 to September 2023 were included. Survival and treatment response were investigated, within the context of the inclusion and exclusion criteria of TOPAZ-1 and in correspondence with genetic alterations of the cancer.
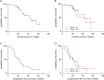
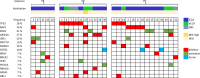
Results: In total, 35 patients, of which 51% met the inclusion criteria of the TOPAZ-1 trial, were analysed. Patients treated within TOPAZ-1 criteria did not have a significantly different median overall survival and progression free survival than the rest of the patients (10.3 versus 9.7 months and 5.3 versus 5 months, respectively). The disease control rate of patients within the TOPAZ-1 criteria was 61.1%, in comparison to 58.8% in the rest of patients. A total of 51 grade 3 and 4 adverse events were observed without significant differences in the subgroups. No specific correlating patterns of genetic alterations with survival and response were observed.
Conclusions: The treatment of advanced patients with BTC with durvalumab and gem/cis, even beyond the inclusion criteria of the TOPAZ-1 trial, shows promising safety.
© 2024. The Author(s).
Conflict of interest statement
P. Michl receives speakers honorary by AstraZeneca. Alexander Olkus, Aurelie Tomczak, Anne Katrin Berger, Conrad Rauber, Philip Puchas, Cyrill Wehling, Thomas Longerich, Arianeb Mehrabi, De-Hua Chang, Jakob Liermann, Sophia Schäfer, Jan Pfeiffenberger, Dirk Jäger, Christoph Springfeld and Michael T. Dill declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

