Age and Computed Tomography and Invasive Coronary Angiography in Stable Chest Pain: A Prespecified Secondary Analysis of the DISCHARGE Randomized Clinical Trial
- PMID: 38416472
- PMCID: PMC10902776
- DOI: 10.1001/jamacardio.2024.0001
Age and Computed Tomography and Invasive Coronary Angiography in Stable Chest Pain: A Prespecified Secondary Analysis of the DISCHARGE Randomized Clinical Trial
Abstract
Importance: The effectiveness and safety of computed tomography (CT) and invasive coronary angiography (ICA) in different age groups is unknown.
Objective: To determine the association of age with outcomes of CT and ICA in patients with stable chest pain.
Design, setting, and participants: The assessor-blinded Diagnostic Imaging Strategies for Patients With Stable Chest Pain and Intermediate Risk of Coronary Artery Disease (DISCHARGE) randomized clinical trial was conducted between October 2015 and April 2019 in 26 European centers. Patients referred for ICA with stable chest pain and an intermediate probability of obstructive coronary artery disease were analyzed in an intention-to-treat analysis. Data were analyzed from July 2022 to January 2023.
Interventions: Patients were randomly assigned to a CT-first strategy or a direct-to-ICA strategy.
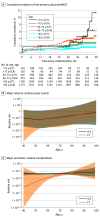
Main outcomes and measures: MACE (ie, cardiovascular death, nonfatal myocardial infarction, or stroke) and major procedure-related complications. The primary prespecified outcome of this secondary analysis of age was major adverse cardiovascular events (MACE) at a median follow-up of 3.5 years.
Results: Among 3561 patients (mean [SD] age, 60.1 [10.1] years; 2002 female [56.2%]), 2360 (66.3%) were younger than 65 years, 982 (27.6%) were between ages 65 to 75 years, and 219 (6.1%) were older than 75 years. The primary outcome was MACE at a median (IQR) follow-up of 3.5 (2.9-4.2) years for 3523 patients (99%). Modeling age as a continuous variable, age, and randomization group were not associated with MACE (hazard ratio, 1.02; 95% CI, 0.98-1.07; P for interaction = .31). Age and randomization group were associated with major procedure-related complications (odds ratio, 1.15; 95% CI, 1.05-1.27; P for interaction = .005), which were lower in younger patients.
Conclusions and relevance: Age did not modify the effect of randomization group on the primary outcome of MACE but did modify the effect on major procedure-related complications. Results suggest that CT was associated with a lower risk of major procedure-related complications in younger patients.
Trial registration: ClinicalTrials.gov Identifier: NCT02400229.
Conflict of interest statement
Figures
References
-
- Haase R, Schlattmann P, Gueret P, et al. ; COME-CCT Consortium . Diagnosis of obstructive coronary artery disease using computed tomography angiography in patients with stable chest pain depending on clinical probability and in clinically important subgroups: meta-analysis of individual patient data. BMJ. 2019;365:l1945. doi:10.1136/bmj.l1945 - DOI - PMC - PubMed
-
- Chang HJ, Lin FY, Gebow D, et al. . Selective referral using CCTA vs direct referral for individuals referred to invasive coronary angiography for suspected CAD: a randomized, controlled, open-label trial. JACC Cardiovasc Imaging. 2019;12(7 Pt 2):1303-1312. doi:10.1016/j.jcmg.2018.09.018 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

