o-Vanillin binds covalently to MAL/TIRAP Lys-210 but independently inhibits TLR2
- PMID: 38416868
- PMCID: PMC10903754
- DOI: 10.1080/14756366.2024.2313055
o-Vanillin binds covalently to MAL/TIRAP Lys-210 but independently inhibits TLR2
Abstract
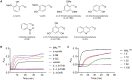
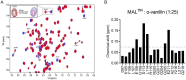
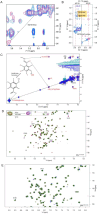
Toll-like receptor (TLR) innate immunity signalling protects against pathogens, but excessive or prolonged signalling contributes to a range of inflammatory conditions. Structural information on the TLR cytoplasmic TIR (Toll/interleukin-1 receptor) domains and the downstream adaptor proteins can help us develop inhibitors targeting this pathway. The small molecule o-vanillin has previously been reported as an inhibitor of TLR2 signalling. To study its mechanism of action, we tested its binding to the TIR domain of the TLR adaptor MAL/TIRAP (MALTIR). We show that o-vanillin binds to MALTIR and inhibits its higher-order assembly in vitro. Using NMR approaches, we show that o-vanillin forms a covalent bond with lysine 210 of MAL. We confirm in mouse and human cells that o-vanillin inhibits TLR2 but not TLR4 signalling, independently of MAL, suggesting it may covalently modify TLR2 signalling complexes directly. Reactive aldehyde-containing small molecules such as o-vanillin may target multiple proteins in the cell.
Keywords: Covalent modification; MyD88 adaptor-like (MAL); Toll-like receptor (TLR); Toll/interleukin-1 receptor domain-containing adaptor protein (TIRAP); nuclear magnetic resonance (NMR); o-vanillin.
Conflict of interest statement
The authors report no conflicts of interest.
Figures

Similar articles
-
Molecular analysis of the binding mode of Toll/interleukin-1 receptor (TIR) domain proteins during TLR2 signaling.Mol Immunol. 2012 Oct;52(3-4):108-16. doi: 10.1016/j.molimm.2012.05.003. Epub 2012 Jun 4. Mol Immunol. 2012. PMID: 22673208
-
Crystal structure of Toll-like receptor adaptor MAL/TIRAP reveals the molecular basis for signal transduction and disease protection.Proc Natl Acad Sci U S A. 2011 Sep 6;108(36):14879-84. doi: 10.1073/pnas.1104780108. Epub 2011 Aug 22. Proc Natl Acad Sci U S A. 2011. PMID: 21873236 Free PMC article.
-
MyD88 adapter-like (Mal)/TIRAP interaction with TRAF6 is critical for TLR2- and TLR4-mediated NF-kappaB proinflammatory responses.J Biol Chem. 2009 Sep 4;284(36):24192-203. doi: 10.1074/jbc.M109.023044. Epub 2009 Jul 10. J Biol Chem. 2009. PMID: 19592497 Free PMC article.
-
Microbial recognition by Toll-like receptors.J Dermatol Sci. 2004 Apr;34(2):73-82. doi: 10.1016/j.jdermsci.2003.10.002. J Dermatol Sci. 2004. PMID: 15033189 Review.
-
Mal and MyD88: adapter proteins involved in signal transduction by Toll-like receptors.J Endotoxin Res. 2003;9(1):55-9. doi: 10.1179/096805103125001351. J Endotoxin Res. 2003. PMID: 12691620 Review.
Cited by
-
Structural characterization of TIR-domain signalosomes through a combination of structural biology approaches.IUCrJ. 2024 Sep 1;11(Pt 5):695-707. doi: 10.1107/S2052252524007693. IUCrJ. 2024. PMID: 39190506 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
