Rare SH2B3 coding variants in lupus patients impair B cell tolerance and predispose to autoimmunity
- PMID: 38417019
- PMCID: PMC10901239
- DOI: 10.1084/jem.20221080
Rare SH2B3 coding variants in lupus patients impair B cell tolerance and predispose to autoimmunity
Abstract
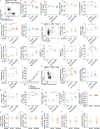
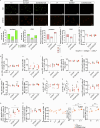
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease with a clear genetic component. While most SLE patients carry rare gene variants in lupus risk genes, little is known about their contribution to disease pathogenesis. Amongst them, SH2B3-a negative regulator of cytokine and growth factor receptor signaling-harbors rare coding variants in over 5% of SLE patients. Here, we show that unlike the variant found exclusively in healthy controls, SH2B3 rare variants found in lupus patients are predominantly hypomorphic alleles, failing to suppress IFNGR signaling via JAK2-STAT1. The generation of two mouse lines carrying patients' variants revealed that SH2B3 is important in limiting the number of immature and transitional B cells. Furthermore, hypomorphic SH2B3 was shown to impair the negative selection of immature/transitional self-reactive B cells and accelerate autoimmunity in sensitized mice, at least in part due to increased IL-4R signaling and BAFF-R expression. This work identifies a previously unappreciated role for SH2B3 in human B cell tolerance and lupus risk.
© 2024 Zhang et al.
Conflict of interest statement
Disclosures: J.C. Lee reported grants from GSK and personal fees from PredictImmune, C4X Discovery, AGPLUS Diagnostics, and Abbvie outside the submitted work. S.L. Masters is a scientific advisor for Odyssey Therapeutics and NRG Therapeutics. No other disclosures were reported.
Figures










References
-
- Alcina, A., Vandenbroeck K., Otaegui D., Saiz A., Gonzalez J.R., Fernandez O., Cavanillas M.L., Cénit M.C., Arroyo R., Alloza I., et al. 2010. The autoimmune disease-associated KIF5A, CD226, and SH2B3 gene variants confer susceptibility for multiple sclerosis. Genes Immun. 11:439–445. 10.1038/gene.2010.30 - DOI - PubMed
-
- Almeida, A.R.M., Neto J.L., Cachucho A., Euzébio M., Meng X., Kim R., Fernandes M.B., Raposo B., Oliveira M.L., Ribeiro D., et al. 2021. Interleukin-7 receptor α mutational activation can initiate precursor B-cell acute lymphoblastic leukemia. Nat. Commun. 12:7268. 10.1038/s41467-021-27197-5 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

