Anti-PD-1 therapy triggers Tfh cell-dependent IL-4 release to boost CD8 T cell responses in tumor-draining lymph nodes
- PMID: 38417020
- PMCID: PMC10901238
- DOI: 10.1084/jem.20232104
Anti-PD-1 therapy triggers Tfh cell-dependent IL-4 release to boost CD8 T cell responses in tumor-draining lymph nodes
Abstract
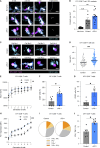
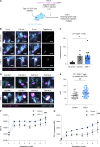
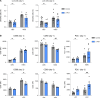
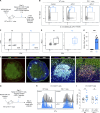
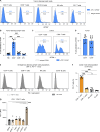
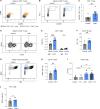
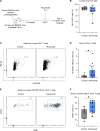
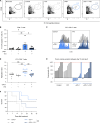
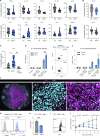
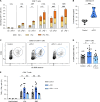
Anti-PD-1 therapy targets intratumoral CD8+ T cells to promote clinical responses in cancer patients. Recent evidence suggests an additional activity in the periphery, but the underlying mechanism is unclear. Here, we show that anti-PD-1 mAb enhances CD8+ T cell responses in tumor-draining lymph nodes by stimulating cytokine production in follicular helper T cells (Tfh). In two different models, anti-PD-1 mAb increased the activation and proliferation of tumor-specific T cells in lymph nodes. Surprisingly, anti-PD-1 mAb did not primarily target CD8+ T cells but instead stimulated IL-4 production by Tfh cells, the major population bound by anti-PD-1 mAb. Blocking IL-4 or inhibiting the Tfh master transcription factor BCL6 abrogated anti-PD-1 mAb activity in lymph nodes while injection of IL-4 complexes was sufficient to recapitulate anti-PD-1 mAb activity. A similar mechanism was observed in a vaccine model. Finally, nivolumab also boosted human Tfh cells in humanized mice. We propose that Tfh cells and IL-4 play a key role in the peripheral activity of anti-PD-1 mAb.
© 2024 Ruggiu et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures



References
-
- Arlauckas, S.P., Garris C.S., Kohler R.H., Kitaoka M., Cuccarese M.F., Yang K.S., Miller M.A., Carlson J.C., Freeman G.J., Anthony R.M., et al. . 2017. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci. Transl. Med. 9:eaal3604. 10.1126/scitranslmed.aal3604 - DOI - PMC - PubMed
-
- Armand, P., Shipp M.A., Ribrag V., Michot J.M., Zinzani P.L., Kuruvilla J., Snyder E.S., Ricart A.D., Balakumaran A., Rose S., and Moskowitz C.H.. 2016. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J. Clin. Oncol. 34:3733–3739. 10.1200/JCO.2016.67.3467 - DOI - PMC - PubMed
-
- Boutsikou, E., Domvri K., Hardavella G., Tsiouda D., Zarogoulidis K., and Kontakiotis T.. 2018. Tumour necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: A pragmatic approach in clinical practice. Ther. Adv. Med. Oncol. 10:1758835918768238. 10.1177/1758835918768238 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

