Current and Emerging Antiviral Agents in the Prevention and Treatment of Cytomegalovirus in Pediatric Transplant Recipients
- PMID: 38417084
- PMCID: PMC10901473
- DOI: 10.1093/jpids/piad059
Current and Emerging Antiviral Agents in the Prevention and Treatment of Cytomegalovirus in Pediatric Transplant Recipients
Abstract
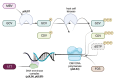
Despite current prophylaxis regimens, cytomegalovirus (CMV) is common in hematopoietic cell transplantation (HCT) and solid organ transplantation (SOT) and remains a significant cause of morbidity and mortality. Newer antiviral medications are reshaping the landscape for prevention and treatment of CMV DNAemia, infection, and disease. Letermovir is approved for CMV prevention in adult HCT patients and is attractive due to the absence of marrow suppression seen with ganciclovir/valganciclovir. Letermovir should not be routinely used for CMV treatment due to its low threshold for resistance. Maribavir is approved for the treatment of refractory or resistant CMV disease in HCT and SOT recipients ≥12 years of age, though it has no current role in CMV prevention. More research is needed to fully elucidate the roles, efficacy, and safety of these newer agents in prevention and treatment of CMV in pediatric transplant recipients.
Keywords: antiviral; cytomegalovirus; pediatric; resistance; transplantation.
© The Author(s) 2024. Published by Oxford University Press on behalf of The Journal of the Pediatric Infectious Diseases Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Deray KGV, Hosek KE, Chilukuri D, et al. Epidemiology and long-term outcomes of cytomegalovirus DNAemia and disease in pediatric solid organ transplant recipients. Am J Transplant 2022; 22:187–98. - PubMed
-
- Downes KJ, Sharova A, Boge CLK, et al. CMV infection and management among pediatric solid organ transplant recipients. Pediatr Transplant 2022; 26:e14220. - PubMed
-
- Wattles BA, Kim AJ, Cheerva AC, Lucas KG, Elder JJ.. Cytomegalovirus treatment in pediatric hematopoietic stem cell transplant patients. J Pediatr Hematol Oncol 2017; 39:241–8. - PubMed
-
- Hakki M, Aitken SL, Danziger-Isakov L, et al. American Society for Transplantation and Cellular Therapy Series: #3—Prevention of cytomegalovirus infection and disease after hematopoietic cell transplantation—ScienceDirect [Internet]. [cited 2023 May 4]. https://www.sciencedirect.com/science/article/pii/S2666636721008927?via%... - PubMed
-
- Kotton CN, Kumar D, Caliendo AM, et al. ; The Transplantation Society International CMV Consensus Group. The Third International Consensus Guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2018; 102:900–31. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

