Unravelling the Oral-Gut Axis: Interconnection Between Periodontitis and Inflammatory Bowel Disease, Current Challenges, and Future Perspective
- PMID: 38417137
- PMCID: PMC11324343
- DOI: 10.1093/ecco-jcc/jjae028
Unravelling the Oral-Gut Axis: Interconnection Between Periodontitis and Inflammatory Bowel Disease, Current Challenges, and Future Perspective
Abstract
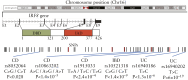
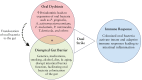
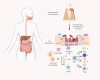
As the opposite ends of the orodigestive tract, the oral cavity and the intestine share anatomical, microbial, and immunological ties that have bidirectional health implications. A growing body of evidence suggests an interconnection between oral pathologies and inflammatory bowel disease [IBD], implying a shift from the traditional concept of independent diseases to a complex, reciprocal cycle. This review outlines the evidence supporting an 'oral-gut' axis, marked by a higher prevalence of periodontitis and other oral conditions in IBD patients and vice versa. We present an in-depth examination of the interconnection between oral pathologies and IBD, highlighting the shared microbiological and immunological pathways, and proposing a 'multi-hit' hypothesis in the pathogenesis of periodontitis-mediated intestinal inflammation. Furthermore, the review underscores the critical need for a collaborative approach between dentists and gastroenterologists to provide holistic oral-systemic healthcare.
Keywords: Crohn’s disease; Periodontitis; dysbiosis; gum–gut axis; immune response; inflammatory bowel disease [IBD]; oral bacteria; oral–gut axis; ulcerative colitis.
Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation (ECCO) 2024. This work is written by US Government employee and is in the public domain in the US.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- D’Souza RN, Collins FS, Murthy VH.. Oral health for all - realizing the promise of science. N Engl J Med 2022;386:809–11. - PubMed
-
- Somerman M, Mouradian WE.. Integrating oral and systemic health: innovations in transdisciplinary science, health care and policy. Frontiers in Dental Medicine 2021;1:674329.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

