Conformational Preference of Lithium Polysulfide Clusters Li2Sx (x = 4-8) in Lithium-Sulfur Batteries
- PMID: 38417153
- PMCID: PMC10934799
- DOI: 10.1021/acs.inorgchem.3c04537
Conformational Preference of Lithium Polysulfide Clusters Li2Sx (x = 4-8) in Lithium-Sulfur Batteries
Abstract
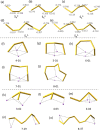
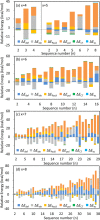
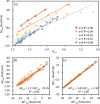
Structures are of fundamental importance for diverse studies of lithium polysulfide clusters, which govern the performance of lithium-sulfur batteries. The ring-like geometries were regarded as the most stable structures, but their physical origin remains elusive. In this work, we systematically explored the minimal structures of Li2Sx (x = 4-8) clusters to uncover the driving force for their conformational preferences. All low-lying isomers were generated by performing global searches using the ABCluster program, and the ionic nature of the Li···S interactions was evidenced with the energy decomposition analysis based on the block-localized wave function (BLW-ED) approach and further confirmed with the quantum theory of atoms in molecule (QTAIM). By analysis of the contributions of various energy components to the relative stability with the references of the lowest-lying isomers, the controlling factor for isomer preferences was found to be the polarization interaction. Notably, although the electrostatic interaction dominates the binding energies, it contributes favorably to the relative stabilities of most isomers. The Li+···Li+ distance is identified as the key geometrical parameter that correlates with the strength of the polarization of the Sx2- fragment imposed by the Li+ cations. Further BLW-ED analyses reveal that the cooperativity of the Li+ cations primarily determines the relative strength of the polarization.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Diao Y.; Xie K.; Xiong S.; Hong X. Shuttle phenomenon — The irreversible oxidation mechanism of sulfur active material in Li-S battery. J. Power Sources 2013, 235, 181–186. 10.1016/j.jpowsour.2013.01.132. - DOI
-
- Zhao E.; Nie K.; Yu X.; Hu Y.; Wang F.; Xiao J.; Li H.; Huang X. Advanced Characterization Techniques in Promoting Mechanism Understanding for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2018, 28 (38), 1707543.10.1002/adfm.201707543. - DOI
-
- Raza H.; Bai S.; Cheng J.; Majumder S.; Zhu H.; Liu Q.; Zheng G.; Li X.; Chen G. Li-S Batteries: Challenges, Achievements and Opportunities. Electrochem. Energy Rev. 2023, 6 (1), 29.10.1007/s41918-023-00188-4. - DOI
LinkOut - more resources
Full Text Sources

