Evaluation of cannabimimetic effects of selected minor cannabinoids and Terpenoids in mice
- PMID: 38417478
- PMCID: PMC11015967
- DOI: 10.1016/j.pnpbp.2024.110984
Evaluation of cannabimimetic effects of selected minor cannabinoids and Terpenoids in mice
Abstract
Background: The cannabis plant contains several cannabinoids, and many terpenoids that give cannabis its distinctive flavoring and aroma. Δ9-Tetrahydrocannabinol (Δ9-THC) is the plant's primary psychoactive constituent. Given the abuse liability of Δ9-THC, assessment of the psychoactive effects of minor cannabinoids and other plant constituents is important, especially for compounds that may be used medicinally. This study sought to evaluate select minor cannabinoids and terpenes for Δ9-THC-like psychoactivity in mouse Δ9-THC drug discrimination and determine their binding affinities at CB1 and CB2 receptors.
Methods: Δ9-THC, cannabidiol (CBD), cannabinol (CBN), cannabichromene (CBC), cannabichromenevarin (CBCV), Δ8-tetrahydrocannabinol (Δ8-THC), (6aR,9R)-Δ10-tetrahydrocannabinol [(6aR,9R)-Δ10-THC], Δ9-tetrahydrocannabinol varin (THCV), β-caryophyllene (BC), and β-caryophyllene oxide (BCO) were examined.
Results: All minor cannabinoids showed measurable cannabinoid 1 (CB1) and cannabinoid 2 (CB2) receptor binding, with CBC, CBCV, and CBD, showing the weakest CB1 receptor binding affinity. BC and BCO exhibited negligible affinity for both CB1 and CB2 receptors. In drug discrimination, only Δ8-THC fully substituted for Δ9-THC, while CBN and (6aR,9R)-Δ10-THC partially substituted for Δ9-THC. THCV and BCO did not alter the discriminative stimulus effects of Δ9-THC.
Conclusion: In summary, only some of myriad cannabinoids and other chemicals found in the cannabis plant bind potently to the identified cannabinoid receptors. Further, only four of the compounds tested herein [Δ9-THC, Δ8-THC, (6aR,9R)-Δ10-THC, and CBN] produced Δ9-THC-like discriminative stimulus effects, suggesting they may possess cannabimimetic subjective effects. Given that the medicinal properties of phytocannabinoids and terpenoids are being investigated scientifically, delineation of their potential adverse effects, including their ability to produce Δ9-THC-like intoxication, is crucial.
Keywords: Cannabidiol; Cannabinol; Drug discrimination; THCV; Tetrahydrocannabinol; β-Caryophyllene.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
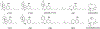

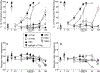
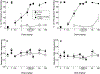
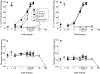
Similar articles
-
Evaluation of prevalent phytocannabinoids in the acetic acid model of visceral nociception.Drug Alcohol Depend. 2009 Nov 1;105(1-2):42-7. doi: 10.1016/j.drugalcdep.2009.06.009. Epub 2009 Aug 12. Drug Alcohol Depend. 2009. PMID: 19679411 Free PMC article.
-
Evaluation of the Modulatory Effects of Minor Cannabinoids and Terpenes on Delta-9-Tetrahydrocannabinol Discrimination in Rats.Cannabis Cannabinoid Res. 2023 Sep;8(S1):S42-S50. doi: 10.1089/can.2023.0062. Cannabis Cannabinoid Res. 2023. PMID: 37721992
-
Cannabidiol attenuates delta 9-tetrahydrocannabinol-like discriminative stimulus effects of cannabinol.Eur J Pharmacol. 1986 Jun 17;125(2):301-4. doi: 10.1016/0014-2999(86)90042-7. Eur J Pharmacol. 1986. PMID: 3017729
-
The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin.Br J Pharmacol. 2008 Jan;153(2):199-215. doi: 10.1038/sj.bjp.0707442. Epub 2007 Sep 10. Br J Pharmacol. 2008. PMID: 17828291 Free PMC article. Review.
-
Synthetic Strategies for Rare Cannabinoids Derived from Cannabis sativa.J Nat Prod. 2022 Jun 24;85(6):1555-1568. doi: 10.1021/acs.jnatprod.2c00155. Epub 2022 Jun 1. J Nat Prod. 2022. PMID: 35648593 Review.
Cited by
-
The Therapeutic Potential of Cannabidiol in the Management of Temporomandibular Disorders and Orofacial Pain.Pharmaceutics. 2025 Mar 3;17(3):328. doi: 10.3390/pharmaceutics17030328. Pharmaceutics. 2025. PMID: 40142992 Free PMC article. Review.
References
-
- Balster RL, Prescott WR, 1992. Δ9-Tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci. Biobehav. Rev 16, 55–62. - PubMed
-
- Barrero AF, Molina J, Enrique Oltra J, Altarejos J, Barragán A, Lara A, Segura M, 1995. Stereochemistry of 14-hydroxy-β-caryophyllene and related compounds. Tetrahedron 51(13), 3813–3822.
-
- Barrett RL, Wiley JL, Balster RL, Martin BR, 1995. Pharmacological specificity of delta 9-tetrahydrocannabinol discrimination in rats. Psychopharmacology (Berl.) 118(4), 419–424. - PubMed
-
- Bueno OF, Carlini EA, Finkelfarb E, Suzuki JS, 1976. Delta 9-Tetrahydrocannabinol, ethanol, and amphetamine as discriminative stimuli-generalization tests with other drugs. Psychopharmacologia 46(3), 235–243. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

