SARS-CoV-2 seroprevalence in vaccine-naïve participants from the Democratic Republic of Congo, Guinea, Liberia, and Mali
- PMID: 38417612
- PMCID: PMC11100347
- DOI: 10.1016/j.ijid.2024.106985
SARS-CoV-2 seroprevalence in vaccine-naïve participants from the Democratic Republic of Congo, Guinea, Liberia, and Mali
Abstract
Objectives: The InVITE study, starting in August 2021, was designed to examine the immunogenicity of different vaccine regimens in several countries including the Democratic Republic of Congo, Guinea, Liberia, and Mali. Prevaccination baseline samples were used to obtain estimates of previous SARS-CoV-2 infection in the study population.
Methods: Adult participants were enrolled upon receipt of their initial COVID-19 vaccine from August 2021 to June 2022. Demographic and comorbidity data were collected at the time of baseline sample collection. SARS-CoV-2 serum anti-Spike and anti-Nucleocapsid antibody levels were measured.
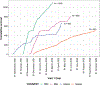
Results: Samples tested included 1016, 375, 663, and 776, from DRC, Guinea, Liberia, and Mali, respectively. Only 0.8% of participants reported a prior positive SARS-CoV-2 test, while 83% and 68% had anti-Spike and anti-Nucleocapsid antibodies, respectively.
Conclusions: Overall SARS-CoV-2 seroprevalence was 86% over the accrual period, suggesting a high prevalence of SARS-CoV-2 infection. Low rates of prior positive test results may be explained by asymptomatic infections, limited access to SARS-CoV-2 test kits and health care, and inadequate surveillance. These seroprevalence rates are from a convenience sample and may not be representative of the population in general, underscoring the need for timely, well-conducted surveillance as part of global pandemic preparedness.
Keywords: COVID-19; InVITE; SARS-CoV-2; Seroprevalence; Vaccines; West Africa.
Copyright © 2024. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
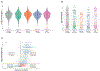
References
-
- WHO. WHO Coronavirus (COVID-19) Dashboard 2023. Available from: https://www.who.int/.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

