Added benefit and revenues of oncology drugs approved by the European Medicines Agency between 1995 and 2020: retrospective cohort study
- PMID: 38418086
- PMCID: PMC10899806
- DOI: 10.1136/bmj-2023-077391
Added benefit and revenues of oncology drugs approved by the European Medicines Agency between 1995 and 2020: retrospective cohort study
Abstract
Objectives: To evaluate the added benefit and revenues of oncology drugs, explore their association, and investigate potential discrepancies between added benefit and revenues across different approval pathways of the European Medicines Agency (EMA).
Design: Retrospective cohort study.
Setting: Oncology drugs and their indications approved by the EMA between 1995 and 2020.
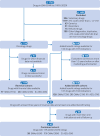
Main outcome measures: Added benefit was evaluated using ratings published by seven organisations: health technology assessment agencies from the United States, France, Germany, and Italy, two medical oncology societies, and a drug bulletin. All retrieved ratings were recategorised using a four point ranking scale to indicate negative or non-quantifiable, minor, substantial, or major added benefit. Revenue data were extracted from publicly available financial reports and compared with published estimates of research and development (R&D) costs. Finally, the association between added benefit and revenue was evaluated. All analyses were performed within the overall study cohort, and within subgroups based on the EMA approval pathway: standard marketing authorisation, conditional marketing authorisation, and authorisation under exceptional circumstances.
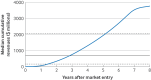
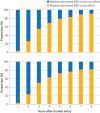
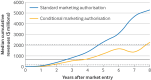
Results: 131 oncology drugs with 166 indications were evaluated for their added benefit by at least one organisation within the required timeframe, yielding a total of 458 added benefit ratings; 189 (41%) were negative or non-quantifiable. The median time to offset the median R&D costs ($684m, £535m, €602m, adjusted to 2020 values) was three years; 50 of 55 (91%) drugs recovered these costs within eight years. Drugs with higher added benefit ratings generally had greater revenues. Negative or non-quantifiable added benefit ratings were more frequent for conditional marketing authorisations and authorisations under exceptional circumstances than for standard marketing authorisations (relative risk 1.53, 95% confidence interval 1.23 to 1.89). Conditional marketing authorisations generated lower revenues and took longer to offset R&D costs than standard marketing authorisations (four years compared with three years).
Conclusions: While revenues seem to align with added benefit, most oncology drugs recover R&D costs within a few years despite providing little added benefit. This is particularly true for drugs approved through conditional marketing authorisations, which inherently appear to lack comprehensive evidence. Policy makers should evaluate whether current regulatory and reimbursement incentives effectively promote development of the most effective drugs for patients with the greatest needs.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://icmje.org/disclosure-of-interest/ and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures

Similar articles
-
Uncertainties about the benefit-risk balance of oncology medicines assessed by the European Medicines Agency.ESMO Open. 2024 Dec;9(12):103991. doi: 10.1016/j.esmoop.2024.103991. Epub 2024 Dec 9. ESMO Open. 2024. PMID: 39657514 Free PMC article.
-
Approval of Cancer Drugs With Uncertain Therapeutic Value: A Comparison of Regulatory Decisions in Europe and the United States.Milbank Q. 2020 Dec;98(4):1219-1256. doi: 10.1111/1468-0009.12476. Epub 2020 Oct 6. Milbank Q. 2020. PMID: 33021339 Free PMC article.
-
Monitoring evidence on overall survival benefits of anticancer drugs approved by the European Medicines Agency between 2009 and 2015.Eur J Cancer. 2019 Mar;110:1-7. doi: 10.1016/j.ejca.2018.12.026. Epub 2019 Feb 5. Eur J Cancer. 2019. PMID: 30735832 Review.
-
Therapeutic Value of Drugs Granted Accelerated Approval or Conditional Marketing Authorization in the US and Europe From 2007 to 2021.JAMA Health Forum. 2022 Aug 5;3(8):e222685. doi: 10.1001/jamahealthforum.2022.2685. JAMA Health Forum. 2022. PMID: 36200635 Free PMC article.
-
[Cancer: Is it really so different? Particularities of oncologic drugs from the perspective of the pharmaceutical regulatory agency].Z Evid Fortbild Qual Gesundhwes. 2013;107(2):120-8. doi: 10.1016/j.zefq.2013.02.003. Epub 2013 Apr 4. Z Evid Fortbild Qual Gesundhwes. 2013. PMID: 23663906 Review. German.
Cited by
-
Why is the market design for innovative pharmaceuticals not well understood?J Comp Eff Res. 2024 Oct;13(10):e240105. doi: 10.57264/cer-2024-0105. Epub 2024 Sep 10. J Comp Eff Res. 2024. PMID: 39254992 Free PMC article. No abstract available.
-
[High-cost medicines: the difficult balance between individual and collective rightsMedicamentos de alto custo: o difícil equilíbrio entre direitos individuais e coletivos].Rev Panam Salud Publica. 2024 Aug 13;48:e76. doi: 10.26633/RPSP.2024.76. eCollection 2024. Rev Panam Salud Publica. 2024. PMID: 39139469 Free PMC article. Spanish.
-
Time-limited reimbursement and Temporary Access Process for early access to oncology treatments in Canada: a perspective based on the epcoritamab experience.J Comp Eff Res. 2025 Jun;14(6):e250024. doi: 10.57264/cer-2025-0024. Epub 2025 Apr 24. J Comp Eff Res. 2025. PMID: 40273150 Free PMC article. Review.
-
Strategies to reduce the cancer burden and improve access to effective and affordable cancer interventions in Europe.Mol Oncol. 2025 Jun;19(6):1553-1560. doi: 10.1002/1878-0261.70058. Epub 2025 May 21. Mol Oncol. 2025. PMID: 40397788 Free PMC article.
-
Expedited Approval in Oncology: A Study of European Regulators' Perspectives and Trade-Offs.Clin Pharmacol Ther. 2025 Sep;118(3):642-648. doi: 10.1002/cpt.3708. Epub 2025 May 13. Clin Pharmacol Ther. 2025. PMID: 40356477 Free PMC article.
References
-
- World Health Organization . Pricing of cancer medicines and its impacts. World Health Organization, 2018.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
