Development of a Simple and Reproducible Cell-derived Orthotopic Xenograft Murine Model for Neuroblastoma
- PMID: 38418146
- PMCID: PMC10905463
- DOI: 10.21873/invivo.13471
Development of a Simple and Reproducible Cell-derived Orthotopic Xenograft Murine Model for Neuroblastoma
Abstract
Background/aim: Neuroblastoma is a common childhood cancer with poor survival for children with high-risk disease, and ongoing research to improve outcomes is needed. Patient-derived xenografts (PDX) and genetically engineered mouse models (GEMM) are reliable models for oncologic research; however, they are resource-intensive, expensive, and require significant expertise to develop and maintain. We developed an orthotopic xenograft murine model of neuroblastoma that utilizes cryopreserved banks of human neuroblastoma cell lines, requires minimal equipment, and is easily reproducible.
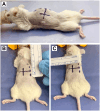
Materials and methods: The neuroblastoma cell line NB1643 was obtained from the Children's Oncology Group (COG) Childhood Cancer Repository. Nod-SCID-gamma (NSG) mice underwent orthotopic injection of 2x106 NB1643 cells suspended in 10 μl of collagen hydrogel directly into the adrenal gland via an open retroperitoneal surgical approach. Mice were monitored by ultrasound and in vivo imaging system (IVIS) until the tumor reached the volume of the ipsilateral kidney. Tumor identity was confirmed by necropsy and histologic analysis.
Results: A total of 55 mice underwent surgery. Eight died due to anesthetic or surgical complications. 39/47 (78%) survivors grew primary adrenal tumors. Average anesthesia time was 30 min. Ultrasound and IVIS successfully characterized tumor growth in all mice. Average time to target tumor size was 5 weeks (range=3-9). Gross pathologic and histologic analysis confirmed adrenal tumors consistent with neuroblastoma in all mice with adrenal masses.
Conclusion: A cell-derived orthotopic xenograft murine model can be successfully used to create an in vivo model of neuroblastoma. This model can be utilized in environments where PDX or GEMM models are not feasible.
Keywords: Neuroblastoma; PDOX; orthotopic murine model.
Copyright © 2024, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors have no conflicts of interest to disclose related to the above manuscript.
Figures








References
-
- VAN Noord RA, Thomas T, Krook M, Chukkapalli S, Hoenerhoff MJ, Dillman JR, Lawlor ER, Opipari VP, Newman EA. Tissue-directed implantation using ultrasound visualization for development of biologically relevant metastatic tumor xenografts. In Vivo. 2017;31(5):779–791. doi: 10.21873/invivo.11131. - DOI - PMC - PubMed
-
- Hidalgo M, Amant F, Biankin AV, Budinská E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Mælandsmo GM, Roman-Roman S, Seoane J, Trusolino L, Villanueva A. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998–1013. doi: 10.1158/2159-8290.CD-14-0001. - DOI - PMC - PubMed
