Functional regulation of aquaporin dynamics by lipid bilayer composition
- PMID: 38418487
- PMCID: PMC10901782
- DOI: 10.1038/s41467-024-46027-y
Functional regulation of aquaporin dynamics by lipid bilayer composition
Abstract
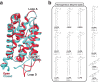
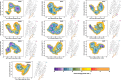
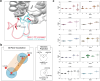
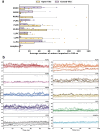
With the diversity of lipid-protein interactions, any observed membrane protein dynamics or functions directly depend on the lipid bilayer selection. However, the implications of lipid bilayer choice are seldom considered unless characteristic lipid-protein interactions have been previously reported. Using molecular dynamics simulation, we characterize the effects of membrane embedding on plant aquaporin SoPIP2;1, which has no reported high-affinity lipid interactions. The regulatory impacts of a realistic lipid bilayer, and nine different homogeneous bilayers, on varying SoPIP2;1 dynamics are examined. We demonstrate that SoPIP2;1's structure, thermodynamics, kinetics, and water transport are altered as a function of each membrane construct's ensemble properties. Notably, the realistic bilayer provides stabilization of non-functional SoPIP2;1 metastable states. Hydrophobic mismatch and lipid order parameter calculations further explain how lipid ensemble properties manipulate SoPIP2;1 behavior. Our results illustrate the importance of careful bilayer selection when studying membrane proteins. To this end, we advise cautionary measures when performing membrane protein molecular dynamics simulations.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Update of
-
Functional Regulation of Aquaporin Dynamics by Lipid Bilayer Composition.bioRxiv [Preprint]. 2023 Jul 22:2023.07.20.549977. doi: 10.1101/2023.07.20.549977. bioRxiv. 2023. Update in: Nat Commun. 2024 Feb 28;15(1):1848. doi: 10.1038/s41467-024-46027-y. PMID: 37502896 Free PMC article. Updated. Preprint.
Similar articles
-
Functional Regulation of Aquaporin Dynamics by Lipid Bilayer Composition.bioRxiv [Preprint]. 2023 Jul 22:2023.07.20.549977. doi: 10.1101/2023.07.20.549977. bioRxiv. 2023. Update in: Nat Commun. 2024 Feb 28;15(1):1848. doi: 10.1038/s41467-024-46027-y. PMID: 37502896 Free PMC article. Updated. Preprint.
-
Surface tension effects on the phase transition of a DPPC bilayer with and without protein: a molecular dynamics simulation.Phys Chem Chem Phys. 2014 May 14;16(18):8434-40. doi: 10.1039/c3cp55524k. Phys Chem Chem Phys. 2014. PMID: 24668218
-
Influence of hydrophobic mismatch on structures and dynamics of gramicidin a and lipid bilayers.Biophys J. 2012 Apr 4;102(7):1551-60. doi: 10.1016/j.bpj.2012.03.014. Epub 2012 Apr 3. Biophys J. 2012. PMID: 22500755 Free PMC article.
-
Elastic deformation and area per lipid of membranes: atomistic view from solid-state deuterium NMR spectroscopy.Biochim Biophys Acta. 2015 Jan;1848(1 Pt B):246-59. doi: 10.1016/j.bbamem.2014.06.004. Epub 2014 Jun 16. Biochim Biophys Acta. 2015. PMID: 24946141 Free PMC article. Review.
-
Exploration of lipid bilayer mechanical properties using molecular dynamics simulation.Arch Biochem Biophys. 2024 Nov;761:110151. doi: 10.1016/j.abb.2024.110151. Epub 2024 Sep 10. Arch Biochem Biophys. 2024. PMID: 39265694 Review.
Cited by
-
Aquaporin Modulation by Cations, a Review.Curr Issues Mol Biol. 2024 Jul 24;46(8):7955-7975. doi: 10.3390/cimb46080470. Curr Issues Mol Biol. 2024. PMID: 39194687 Free PMC article. Review.
-
SWEET family transporters act as water conducting carrier proteins in plants.bioRxiv [Preprint]. 2024 Jun 25:2024.06.23.600272. doi: 10.1101/2024.06.23.600272. bioRxiv. 2024. Update in: J Chem Inf Model. 2025 Apr 14;65(7):3697-3705. doi: 10.1021/acs.jcim.5c00110. PMID: 38979333 Free PMC article. Updated. Preprint.
-
Interplay between phosphorylation and oligomerization tunes the conformational ensemble of SWEET transporters.bioRxiv [Preprint]. 2024 Jun 14:2024.06.12.598708. doi: 10.1101/2024.06.12.598708. bioRxiv. 2024. PMID: 38915650 Free PMC article. Preprint.
-
ESMDynamic: Fast and Accurate Prediction of Protein Dynamic Contact Maps from Single Sequences.bioRxiv [Preprint]. 2025 Aug 24:2025.08.20.671365. doi: 10.1101/2025.08.20.671365. bioRxiv. 2025. PMID: 40894558 Free PMC article. Preprint.
-
Targeting Bacterial RNA Polymerase: Harnessing Simulations and Machine Learning to Design Inhibitors for Drug-Resistant Pathogens.Biochemistry. 2025 Mar 18;64(6):1169-1179. doi: 10.1021/acs.biochem.4c00751. Epub 2025 Feb 27. Biochemistry. 2025. PMID: 40014017 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

