104-week efficacy and safety of cipaglucosidase alfa plus miglustat in adults with late-onset Pompe disease: a phase III open-label extension study (ATB200-07)
- PMID: 38418563
- PMCID: PMC11055775
- DOI: 10.1007/s00415-024-12236-0
104-week efficacy and safety of cipaglucosidase alfa plus miglustat in adults with late-onset Pompe disease: a phase III open-label extension study (ATB200-07)
Abstract
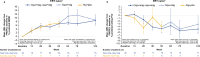
The phase III double-blind PROPEL study compared the novel two-component therapy cipaglucosidase alfa + miglustat (cipa + mig) with alglucosidase alfa + placebo (alg + pbo) in adults with late-onset Pompe disease (LOPD). This ongoing open-label extension (OLE; NCT04138277) evaluates long-term safety and efficacy of cipa + mig. Outcomes include 6-min walk distance (6MWD), forced vital capacity (FVC), creatine kinase (CK) and hexose tetrasaccharide (Hex4) levels, patient-reported outcomes and safety. Data are reported as change from PROPEL baseline to OLE week 52 (104 weeks post-PROPEL baseline). Of 118 patients treated in the OLE, 81 continued cipa + mig treatment from PROPEL (cipa + mig group; 61 enzyme replacement therapy [ERT] experienced prior to PROPEL; 20 ERT naïve) and 37 switched from alg + pbo to cipa + mig (switch group; 29 ERT experienced; 8 ERT naive). Mean (standard deviation [SD]) change in % predicted 6MWD from baseline to week 104 was + 3.1 (8.1) for cipa + mig and - 0.5 (7.8) for the ERT-experienced switch group, and + 8.6 (8.6) for cipa + mig and + 8.9 (11.7) for the ERT-naïve switch group. Mean (SD) change in % predicted FVC was - 0.6 (7.5) for cipa + mig and - 3.8 (6.2) for the ERT-experienced switch group, and - 4.8 (6.5) and - 3.1 (6.7), respectively, in ERT-naïve patients. CK and Hex4 levels improved in both treatment groups by week 104 with cipa + mig treatment. Three patients discontinued the OLE due to infusion-associated reactions. No new safety signals were identified. Cipa + mig treatment up to 104 weeks was associated with overall maintained improvements (6MWD, biomarkers) or stabilization (FVC) from baseline with continued durability, and was well tolerated, supporting long-term benefits for patients with LOPD.Trial registration number: NCT04138277; trial start date: December 18, 2019.
Keywords: n-butyldeoxynojirimycin; Alpha glucosidase; Glycogen storage disease type II; Lysosomal storage disorders; Myozyme.
© 2024. The Author(s).
Conflict of interest statement
Benedikt Schoser has received unrestricted research grants from Amicus Therapeutics Inc., Astellas, Roche, Marigold Foundation, AMDA Foundation and speaker’s honoraria from Amicus Therapeutics Inc., Alexion, Kedrion, Sanofi. He has participated as a scientific advisor for Amicus Therapeutics Inc., Argenx, Astellas, Bayer, Maze, Pepgen, Sanofi, Spark, and Taysha. He declares no stocks or shares. Drago Bratkovic declares no competing interests. Barry J. Byrne has participated as a consultant/advisory board member for Pfizer, Amicus Therapeutics, Inc., and Sanofi. He also owns stocks in Lacerta Therapeutics. Kristl G. Claeys received research funding from Alnylam, Biogen, Pfizer, Roche, Sanofi-Genzyme; received advisory board member honoraria from Alexion, Alnylam, Amicus, ArgenX, Biogen, Ipsen, Janssen Pharmaceutics, Lupin, Pfizer, Roche, Sanofi-Genzyme and UCB and is Chairholder of the Emil von Behring Chair for Neuromuscular and Neurodegenerative Disorders by CSL Behring. Jordi Diaz-Manera has received consulting fees/honoraria from Sarepta, Sanofi and Audentes and grant support from Sanofi, Spark and Boehringer Ingelheim. He has received payment for speaking from Sanofi, Sarepta and Lupin. Priya S. Kishnani has received research/grant support from Sanofi Genzyme and Amicus Therapeutics Inc. and has received consulting fees and honoraria from Sanofi Genzyme, Amicus Therapeutics, Inc., Maze Therapeutics, Bayer and Asklepios Biopharmaceutical, Inc. (AskBio). She is a member of the Pompe and Gaucher Disease Registry Advisory Board for Sanofi Genzyme, Pompe Disease Advisory Board for Amicus Therapeutics Inc., and Advisory Board for Baebies. Priya S. Kishnani has equity with Maze Therapeutics and has held equity in Asklepios Biopharmaceuticals and may receive milestone payments related to that equity in the future. Pascal Laforet is a consultant/advisory board member for Amicus Therapeutics Inc., Sanofi Genzyme and Spark Therapeutics. He has received travel expenses from Sanofi Genzyme and Spark Therapeutics. Mark Roberts has received honorarium for educational symposia from Sanofi Genzyme and Amicus and for participation on advisory boards for Sanofi, and Amicus. Antonio Toscano received honorarium for educational talks from Sanofi Genzyme and Amicus and for participation on advisory boards for Sanofi, Amicus, Aro and Spark. He is a member of the European Reference Network for Neuromuscular Disorders (EU-NMD), Project ID 739543. Ans T. van der Ploeg is an advisory board member of Amicus Therapeutics Inc., BioMarin, Sanofi Genzyme, and Spark Therapeutics. She has provided consultancies for Amicus Therapeutics Inc., BioMarin, Sanofi Genzyme, and Spark Therapeutics and has contracted research for Amicus Therapeutics Inc., BioMarin, Sanofi Genzyme, and Spark Therapeutics. All collaborations are done under an agreement between Erasmus MC and these industries. Tahseen Mozaffar has participated in an advisory capacity for Abbvie, Alexion, Amicus Therapeutics, Inc., Annji, Argenx, Arvinas, Audentes, Cabaletta, Maze Therapeutics, Momenta, Ra Pharmaceuticals, Sanofi Genzyme, Sarepta, Spark Therapeutics, and UCB, and has participated in the speaker’s bureau for Sanofi Genzyme. He is a member of the medical advisory board for the Myositis Association, Neuromuscular Disease Foundation, Myasthenia Gravis Foundation of California and Myasthenia Gravis Foundation of America. He has received research funding from the Myositis Association, the Muscular Dystrophy Association, the NIH and from the following sponsors: Alexion, Amicus Therapeutics, Inc., Annji, Argenx, Audentes, Bristol-Myers Squib, Cabaletta, Cartesian Therapeutics, Grifols, Momenta, Ra Pharmaceuticals, Sanofi Genzyme, Spark Therapeutics, UCB, and Valerion. He is a member of the Data safety monitoring board for Acceleron, Applied Therapeutics, Sarepta, and the NIH. Jeff Castelli, Mitchell Goldman, Fred Holdbrook, Sheela Sitaraman Das, and Yasmine Wasfi are employees of, and hold stocks and shares in, Amicus Therapeutics, Inc.
Figures



References
-
- Cabello J, Marsden D. Pompe disease: clinical perspectives. Orphan Drugs Res Rev. 2017;7:1–10. doi: 10.2147/odrr.S69109. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

