APE1 inhibition enhances ferroptotic cell death and contributes to hepatocellular carcinoma therapy
- PMID: 38418695
- PMCID: PMC11043431
- DOI: 10.1038/s41418-024-01270-0
APE1 inhibition enhances ferroptotic cell death and contributes to hepatocellular carcinoma therapy
Abstract
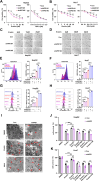
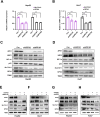
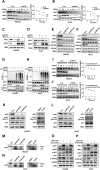
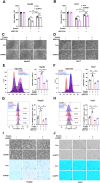
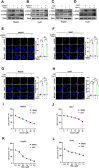
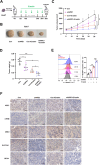
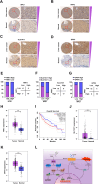
Ferroptosis, a regulated form of cell death triggered by iron-dependent lipid peroxidation, has emerged as a promising therapeutic strategy for cancer treatment, particularly in hepatocellular carcinoma (HCC). However, the mechanisms underlying the regulation of ferroptosis in HCC remain to be unclear. In this study, we have identified a novel regulatory pathway of ferroptosis involving the inhibition of Apurinic/apyrimidinic endonuclease 1 (APE1), a key enzyme with dual functions in DNA repair and redox regulation. Our findings demonstrate that inhibition of APE1 leads to the accumulation of lipid peroxidation and enhances ferroptosis in HCC. At the molecular level, the inhibition of APE1 enhances ferroptosis which relies on the redox activity of APE1 through the regulation of the NRF2/SLC7A11/GPX4 axis. We have identified that both genetic and chemical inhibition of APE1 increases AKT oxidation, resulting in an impairment of AKT phosphorylation and activation, which leads to the dephosphorylation and activation of GSK3β, facilitating the subsequent ubiquitin-proteasome-dependent degradation of NRF2. Consequently, the downregulation of NRF2 suppresses SLC7A11 and GPX4 expression, triggering ferroptosis in HCC cells and providing a potential therapeutic approach for ferroptosis-based therapy in HCC. Overall, our study uncovers a novel role and mechanism of APE1 in the regulation of ferroptosis and highlights the potential of targeting APE1 as a promising therapeutic strategy for HCC and other cancers.
© 2024. The Author(s), under exclusive licence to ADMC Associazione Differenziamento e Morte Cellulare.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Esculetin triggers ferroptosis via inhibition of the Nrf2-xCT/GPx4 axis in hepatocellular carcinoma.Chin J Nat Med. 2025 Apr;23(4):443-456. doi: 10.1016/S1875-5364(25)60853-3. Chin J Nat Med. 2025. PMID: 40274347
-
Picropodophyllin induces ferroptosis via blockage of AKT/NRF2/SLC7A11 and AKT/NRF2/SLC40A1 axes in hepatocellular carcinoma as a natural IGF1R inhibitor.Phytomedicine. 2025 Jul 25;143:156840. doi: 10.1016/j.phymed.2025.156840. Epub 2025 May 10. Phytomedicine. 2025. PMID: 40412057
-
MCM4 potentiates evasion of hepatocellular carcinoma from sorafenib-induced ferroptosis through Nrf2 signaling pathway.Int Immunopharmacol. 2024 Dec 5;142(Pt A):113107. doi: 10.1016/j.intimp.2024.113107. Epub 2024 Sep 13. Int Immunopharmacol. 2024. PMID: 39276458
-
[Research progress on ferroptosis regulation in tumor immunity of hepatocellular carcinoma].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024 Dec 25;53(6):715-725. doi: 10.3724/zdxbyxb-2024-0117. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 39694527 Free PMC article. Review. Chinese.
-
Ferroptosis in liver cancer: a key role of post-translational modifications.Front Immunol. 2024 Apr 8;15:1375589. doi: 10.3389/fimmu.2024.1375589. eCollection 2024. Front Immunol. 2024. PMID: 38650929 Free PMC article. Review.
Cited by
-
Sorafenib-induced macrophage extracellular traps via ARHGDIG/IL4/PADI4 axis confer drug resistance through inhibiting ferroptosis in hepatocellular carcinoma.Biol Direct. 2024 Nov 11;19(1):110. doi: 10.1186/s13062-024-00560-4. Biol Direct. 2024. PMID: 39529192 Free PMC article.
-
Isoliensinine exerts antitumor effects in lung adenocarcinoma by inhibiting APEX1-driven ROS production.Front Pharmacol. 2025 May 27;16:1555802. doi: 10.3389/fphar.2025.1555802. eCollection 2025. Front Pharmacol. 2025. PMID: 40495893 Free PMC article.
-
Current Progress of Ferroptosis Study in Hepatocellular Carcinoma.Int J Biol Sci. 2024 Jul 1;20(9):3621-3637. doi: 10.7150/ijbs.96014. eCollection 2024. Int J Biol Sci. 2024. PMID: 38993573 Free PMC article. Review.
-
A Dual-Targeting Biomimetic Nanoplatform Integrates SDT/CDT/Gas Therapy to Boost Synergistic Ferroptosis for Orthotopic Hepatocellular Carcinoma Therapy.Adv Sci (Weinh). 2025 Feb;12(8):e2413833. doi: 10.1002/advs.202413833. Epub 2025 Jan 9. Adv Sci (Weinh). 2025. PMID: 39783849 Free PMC article.
-
Silencing NRF2 enhances arsenic trioxide-induced ferroptosis in hepatocellular carcinoma cells.PLoS One. 2025 May 22;20(5):e0322746. doi: 10.1371/journal.pone.0322746. eCollection 2025. PLoS One. 2025. PMID: 40402956 Free PMC article.
References
-
- Lang X, Green MD, Wang W, Yu J, Choi JE, Jiang L, et al. Radiotherapy and Immunotherapy Promote Tumoral Lipid Oxidation and Ferroptosis via Synergistic Repression of SLC7A11. Cancer Discov. 2019;9:1673–85. doi: 10.1158/2159-8290.CD-19-0338. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

