Low pressure reversibly driving colossal barocaloric effect in two-dimensional vdW alkylammonium halides
- PMID: 38418810
- PMCID: PMC10901796
- DOI: 10.1038/s41467-024-46248-1
Low pressure reversibly driving colossal barocaloric effect in two-dimensional vdW alkylammonium halides
Abstract
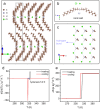
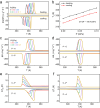
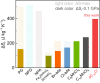
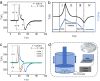
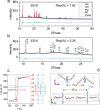
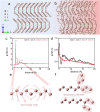
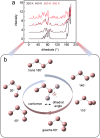
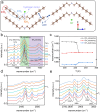
Plastic crystals as barocaloric materials exhibit the large entropy change rivalling freon, however, the limited pressure-sensitivity and large hysteresis of phase transition hinder the colossal barocaloric effect accomplished reversibly at low pressure. Here we report reversible colossal barocaloric effect at low pressure in two-dimensional van-der-Waals alkylammonium halides. Via introducing long carbon chains in ammonium halide plastic crystals, two-dimensional structure forms in (CH3-(CH2)n-1)2NH2X (X: halogen element) with weak interlayer van-der-Waals force, which dictates interlayer expansion as large as 13% and consequently volume change as much as 12% during phase transition. Such anisotropic expansion provides sufficient space for carbon chains to undergo dramatic conformation disordering, which induces colossal entropy change with large pressure-sensitivity and small hysteresis. The record reversible colossal barocaloric effect with entropy change ΔSr ~ 400 J kg-1 K-1 at 0.08 GPa and adiabatic temperature change ΔTr ~ 11 K at 0.1 GPa highlights the design of novel barocaloric materials by engineering the dimensionality of plastic crystals.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Colossal barocaloric effects with ultralow hysteresis in two-dimensional metal-halide perovskites.Nat Commun. 2022 May 9;13(1):2536. doi: 10.1038/s41467-022-29800-9. Nat Commun. 2022. PMID: 35534457 Free PMC article.
-
Colossal Barocaloric Effect in Liquid Crystals via Cascade Order-Disorder Transitions.ACS Appl Mater Interfaces. 2025 Jul 30;17(30):43158-43168. doi: 10.1021/acsami.5c10519. Epub 2025 Jul 17. ACS Appl Mater Interfaces. 2025. PMID: 40673771
-
Dynamics in the ordered and disordered phases of barocaloric adamantane.Phys Chem Chem Phys. 2023 Mar 29;25(13):9282-9293. doi: 10.1039/d2cp05412d. Phys Chem Chem Phys. 2023. PMID: 36919868
-
Giant and Reversible Barocaloric Effect in Trinuclear Spin-Crossover Complex Fe3 (bntrz)6 (tcnset)6.Adv Mater. 2021 Mar;33(10):e2008076. doi: 10.1002/adma.202008076. Epub 2021 Feb 2. Adv Mater. 2021. PMID: 33527567 Review.
-
High Entropy van der Waals Materials.Adv Sci (Weinh). 2022 Oct;9(30):e2203219. doi: 10.1002/advs.202203219. Epub 2022 Aug 25. Adv Sci (Weinh). 2022. PMID: 36008123 Free PMC article. Review.
Cited by
-
All-temperature barocaloric effects at pressure-induced phase transitions.Nat Commun. 2025 Aug 19;16(1):7713. doi: 10.1038/s41467-025-63068-z. Nat Commun. 2025. PMID: 40830339 Free PMC article.
References
-
- Hu F, et al. Influence of negative lattice expansion and metamagnetic transition on magnetic entropy change in the compound LaFe11.4Si1.6. Appl. Phys. Lett. 2001;78:3675–3677. doi: 10.1063/1.1375836. - DOI
LinkOut - more resources
Full Text Sources

