Diminished NAD+ levels and activation of retrotransposons promote postovulatory aged oocyte (POAO) death
- PMID: 38418811
- PMCID: PMC10902361
- DOI: 10.1038/s41420-024-01876-w
Diminished NAD+ levels and activation of retrotransposons promote postovulatory aged oocyte (POAO) death
Abstract
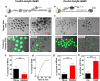
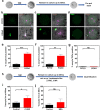
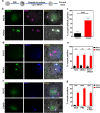
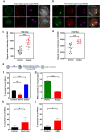
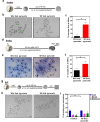
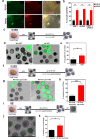
Death is the fate of postovulatory aged or unfertilized oocytes (POAO) in many animals. However, precise molecular mechanisms are yet to be discovered. Here, we demonstrate that increased amounts of reactive oxygen species (ROS), calcium ion (Ca+2) channels, and retrotransposon activity induce apoptosis, which in turn causes POAO death. Notably, suppression of ROS, Ca+2 channels, and retrotransposons delayed POAO death. Further, we found that the histone H4K12 and K16 acetylation increased via downregulation of NAD+ and NAD+ -dependent histone deacetylase SIRT3. Furthermore, adding NMN, sodium pyruvate, or CD38 inhibition delayed the death of postovulatory aged oocytes. Finally, we demonstrate the conservation of retrotransposon-induced DNA damage-dependent POAO death in higher-order vertebrates. Our findings suggest that POAO mortality is caused by cyclic cascade metabolic interactions in which low NAD+ levels increase histone acetylation by inhibiting histone deacetylases, resulting in an increase in retrotransposons, ROS, and Ca+2 channel activity and thus contributing to DNA damage-induced apoptosis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Holesh JE, Bass AN, Lord M. Physiology, Ovulation. Stat Pearls [Internet]. Treasure Island (FL): Stat Pearls Publishing. 2022.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

