Multisensory gamma stimulation promotes glymphatic clearance of amyloid
- PMID: 38418876
- PMCID: PMC10917684
- DOI: 10.1038/s41586-024-07132-6
Multisensory gamma stimulation promotes glymphatic clearance of amyloid
Abstract
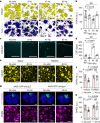
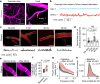
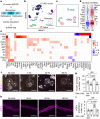
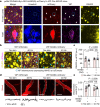
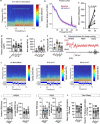
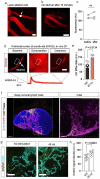
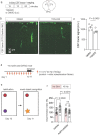
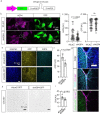
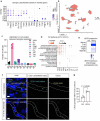
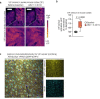
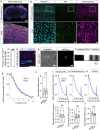
The glymphatic movement of fluid through the brain removes metabolic waste1-4. Noninvasive 40 Hz stimulation promotes 40 Hz neural activity in multiple brain regions and attenuates pathology in mouse models of Alzheimer's disease5-8. Here we show that multisensory gamma stimulation promotes the influx of cerebrospinal fluid and the efflux of interstitial fluid in the cortex of the 5XFAD mouse model of Alzheimer's disease. Influx of cerebrospinal fluid was associated with increased aquaporin-4 polarization along astrocytic endfeet and dilated meningeal lymphatic vessels. Inhibiting glymphatic clearance abolished the removal of amyloid by multisensory 40 Hz stimulation. Using chemogenetic manipulation and a genetically encoded sensor for neuropeptide signalling, we found that vasoactive intestinal peptide interneurons facilitate glymphatic clearance by regulating arterial pulsatility. Our findings establish novel mechanisms that recruit the glymphatic system to remove brain amyloid.
© 2024. The Author(s).
Conflict of interest statement
L.-H.T. and E.S.B. are co-scientific founders and serve on the scientific advisory board of Cognito Therapeutics. The other authors declare no competing interests.
Figures






Comment in
-
Neuronal activity drives glymphatic waste clearance.Nat Rev Neurol. 2024 May;20(5):255. doi: 10.1038/s41582-024-00963-x. Nat Rev Neurol. 2024. PMID: 38622282 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

