Neuronal dynamics direct cerebrospinal fluid perfusion and brain clearance
- PMID: 38418877
- PMCID: PMC12054998
- DOI: 10.1038/s41586-024-07108-6
Neuronal dynamics direct cerebrospinal fluid perfusion and brain clearance
Abstract
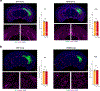
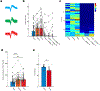
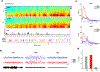
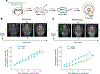
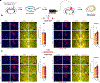
The accumulation of metabolic waste is a leading cause of numerous neurological disorders, yet we still have only limited knowledge of how the brain performs self-cleansing. Here we demonstrate that neural networks synchronize individual action potentials to create large-amplitude, rhythmic and self-perpetuating ionic waves in the interstitial fluid of the brain. These waves are a plausible mechanism to explain the correlated potentiation of the glymphatic flow1,2 through the brain parenchyma. Chemogenetic flattening of these high-energy ionic waves largely impeded cerebrospinal fluid infiltration into and clearance of molecules from the brain parenchyma. Notably, synthesized waves generated through transcranial optogenetic stimulation substantially potentiated cerebrospinal fluid-to-interstitial fluid perfusion. Our study demonstrates that neurons serve as master organizers for brain clearance. This fundamental principle introduces a new theoretical framework for the functioning of macroscopic brain waves.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures
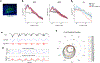
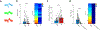








Comment in
-
Neuronal activity drives glymphatic waste clearance.Nat Rev Neurol. 2024 May;20(5):255. doi: 10.1038/s41582-024-00963-x. Nat Rev Neurol. 2024. PMID: 38622282 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

