Compartmentalized ocular lymphatic system mediates eye-brain immunity
- PMID: 38418880
- PMCID: PMC10990932
- DOI: 10.1038/s41586-024-07130-8
Compartmentalized ocular lymphatic system mediates eye-brain immunity
Abstract
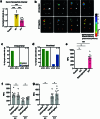
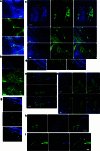
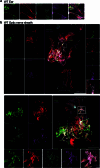
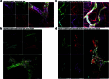
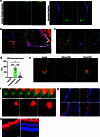
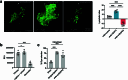
The eye, an anatomical extension of the central nervous system (CNS), exhibits many molecular and cellular parallels to the brain. Emerging research demonstrates that changes in the brain are often reflected in the eye, particularly in the retina1. Still, the possibility of an immunological nexus between the posterior eye and the rest of the CNS tissues remains unexplored. Here, studying immune responses to herpes simplex virus in the brain, we observed that intravitreal immunization protects mice against intracranial viral challenge. This protection extended to bacteria and even tumours, allowing therapeutic immune responses against glioblastoma through intravitreal immunization. We further show that the anterior and posterior compartments of the eye have distinct lymphatic drainage systems, with the latter draining to the deep cervical lymph nodes through lymphatic vasculature in the optic nerve sheath. This posterior lymphatic drainage, like that of meningeal lymphatics, could be modulated by the lymphatic stimulator VEGFC. Conversely, we show that inhibition of lymphatic signalling on the optic nerve could overcome a major limitation in gene therapy by diminishing the immune response to adeno-associated virus and ensuring continued efficacy after multiple doses. These results reveal a shared lymphatic circuit able to mount a unified immune response between the posterior eye and the brain, highlighting an understudied immunological feature of the eye and opening up the potential for new therapeutic strategies in ocular and CNS diseases.
© 2024. The Author(s).
Conflict of interest statement
E.S., A.R. and A.I. are co-founders of Rho Bio. A.I. is a member of the Board of Directors of Roche Holding Ltd. E.S., J.-L.T. and A.I. are co-inventors on patent application No. 62/929,527, “Manipulation of meningeal lymphatic vasculature for brain and CNS tumour therapy”. W.M.S. is a co-founder of B3 Therapeutics, Stradefy and Xanadu Bio. W.M.S. is a consultant to Xanadu Bio, Stradefy Biosciences, Johnson & Johnson, Celanese, Cranius and CMC Pharma.
Figures








References
-
- London A, Benhar I, Schwartz M. The retina as a window to the brain—from eye research to CNS disorders. Nat. Rev. Neurol. 2013;9:44–53. - PubMed
-
- Marcocci ME, et al. Herpes simplex virus-1 in the brain: the dark side of a sneaky infection. Trends Microbiol. 2020;28:808–820. - PubMed
-
- Victora GD, Nussenzweig MC. Germinal centers. Annu. Rev. Immunol. 2022;40:413–442. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

