Crym-positive striatal astrocytes gate perseverative behaviour
- PMID: 38418885
- PMCID: PMC10937394
- DOI: 10.1038/s41586-024-07138-0
Crym-positive striatal astrocytes gate perseverative behaviour
Abstract
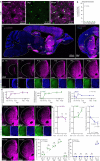
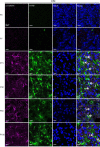
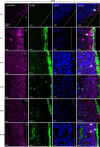
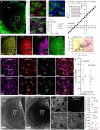
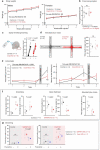
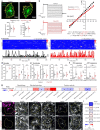
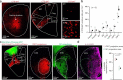
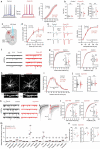
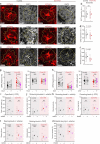
Astrocytes are heterogeneous glial cells of the central nervous system1-3. However, the physiological relevance of astrocyte diversity for neural circuits and behaviour remains unclear. Here we show that a specific population of astrocytes in the central striatum expresses μ-crystallin (encoded by Crym in mice and CRYM in humans) that is associated with several human diseases, including neuropsychiatric disorders4-7. In adult mice, reducing the levels of μ-crystallin in striatal astrocytes through CRISPR-Cas9-mediated knockout of Crym resulted in perseverative behaviours, increased fast synaptic excitation in medium spiny neurons and dysfunctional excitatory-inhibitory synaptic balance. Increased perseveration stemmed from the loss of astrocyte-gated control of neurotransmitter release from presynaptic terminals of orbitofrontal cortex-striatum projections. We found that perseveration could be remedied using presynaptic inhibitory chemogenetics8, and that this treatment also corrected the synaptic deficits. Together, our findings reveal converging molecular, synaptic, circuit and behavioural mechanisms by which a molecularly defined and allocated population of striatal astrocytes gates perseveration phenotypes that accompany neuropsychiatric disorders9-12. Our data show that Crym-positive striatal astrocytes have key biological functions within the central nervous system, and uncover astrocyte-neuron interaction mechanisms that could be targeted in treatments for perseveration.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

