Independent clinic-based evaluation of dual POCTs for screening for HIV and syphilis in men who have sex with men in Italy, Malta, Peru, and the United Kingdom
- PMID: 38418941
- PMCID: PMC10902927
- DOI: 10.1186/s12879-024-09019-3
Independent clinic-based evaluation of dual POCTs for screening for HIV and syphilis in men who have sex with men in Italy, Malta, Peru, and the United Kingdom
Abstract
Introduction: Globally, the incidence of HIV and syphilis can be reduced by the use of validated point of care tests (POCTs). As part of the WHO PRoSPeRo Network, we aimed to evaluate the performance, acceptability, and operational characteristics of two dual HIV/syphilis POCTs (Bioline HIV/Syphilis Duo (Abbott) and DPP® HIV-Syphilis assay (Chembio) for the screening of HIV and syphilis amongst men who have sex with men (MSM).
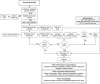
Method and analyses: A cross sectional study of 2,577 MSM in Italy, Malta, Peru, and the United Kingdom (UK) presenting to seven clinic sites, were enrolled. Finger prick blood was collected to perform POCTs and results compared with standard laboratory investigations on venepuncture blood. Acceptability and operational characteristics were assessed using questionnaires. Diagnostic meta-analysis was used to combine data from the evaluation sites.
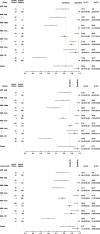
Results: Based on laboratory tests, 23.46% (n = 598/2549) of participants were confirmed HIV positive, and 35.88% of participants (n = 901/2511) were positive on treponemal reference testing. Of all participants showing evidence of antibodies to Treponema pallidum, 50.56% (n = 455/900) were Rapid Plasma Reagin (RPR) test reactive. Of HIV positive individuals, 60.62% (n = 354/584) had evidence of antibodies to T. pallidum, and of these 60.45% (n = 214/354) exhibited reactive RPR tests indicating probable (co)infection. For Bioline POCT, pooled sensitivities and specificities for HIV were 98.95% and 99.89% respectively, and for syphilis were 73.79% and 99.57%. For Chembio pooled sensitivities and specificities for HIV were 98.66% and 99.55%, and for syphilis were 78.60% and 99.48%. Both tests can detect greater than 90% of probable active syphilis cases, as defined by reactive RPR and treponemal test results. These dual POCTs were preferred by 74.77% (n = 1,926) of participants, due to their convenience, and the operational characteristics made them acceptable to health care providers (HCPs).
Conclusions: Both the Bioline and the Chembio dual POCT for syphilis and HIV had acceptable performance, acceptability and operational characteristics amongst MSM in the PRoSPeRo network. These dual POCTs could serve as a strategic, more cost effective, patient and healthcare provider (HCP) friendly alternative to conventional testing; in clinical and other field settings, especially those in resource-limited settings.
Keywords: Clinic-based evaluation; HIV; Men who have Sex with Men; Point-of-Care-Tests; Public Health; Syphilis.
© 2024. The Author(s).
Conflict of interest statement
The POCT manufacturers disclose and furnish free of charge to WHO the information and sufficient quantities of the product(s) in order to enable this evaluation as part of the WHO/RHR STI POC initiative. WHO is entitled to evaluate and publish the trial results, and to exclusively control this evaluation and the content of the aforesaid publication. WHO shall submit any proposed publication to the manufacturers for review, comments received will be considered in good faith, but the decision to publish rests with WHO.
Figures


References
-
- World Health Organization. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021: accountability for the global health sector strategies 2016–2021. Geneva; 2021.
-
- World Health Organization. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021: accountability for the global health sector strategies 2016–2021 - Web Annex 2: data methods. Geneva; 2021
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

