The effect of combining an inhaled corticosteroid and a long-acting muscarinic antagonist on human airway epithelial cells in vitro
- PMID: 38419021
- PMCID: PMC10902985
- DOI: 10.1186/s12931-024-02710-8
The effect of combining an inhaled corticosteroid and a long-acting muscarinic antagonist on human airway epithelial cells in vitro
Abstract
Background: Airway epithelial cells (AECs) are a major component of local airway immune responses. Direct effects of type 2 cytokines on AECs are implicated in type 2 asthma, which is driven by epithelial-derived cytokines and leads to airway obstruction. However, evidence suggests that restoring epithelial health may attenuate asthmatic features.
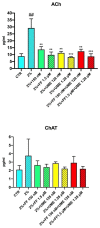
Methods: We investigated the effects of passive sensitisation on IL-5, NF-κB, HDAC-2, ACh, and ChAT in human bronchial epithelial cells (HBEpCs) and the effects of fluticasone furoate (FF) and umeclidinium (UME) alone and in combination on these responses.
Results: IL-5 and NF-κB levels were increased, and that of HDAC-2 reduced in sensitised HEBpCs. Pretreatment with FF reversed the effects of passive sensitisation by concentration-dependent reduction of IL-5, resulting in decreased NF-κB levels and restored HDAC-2 activity. Addition of UME enhanced these effects. Sensitized HEBpCs also exhibited higher ACh and ChAT levels. Pretreatment with UME significantly reduced ACh levels, and addition of FF caused a further small reduction.
Conclusion: This study confirmed that passive sensitisation of AECs results in an inflammatory response with increased levels of IL-5 and NF-κB, reduced levels of HDAC-2, and higher levels of ACh and ChAT compared to normal cells. Combining FF and UME was found to be more effective in reducing IL-5, NF-κB, and ACh and restoring HDAC-2 compared to the individual components. This finding supports adding a LAMA to established ICS/LABA treatment in asthma and suggests the possibility of using an ICS/LAMA combination when needed.
Keywords: Airway epithelial cells; Asthma; Inflammation; Inhaled corticosteroid; Long-acting muscarinic antagonists.
© 2024. The Author(s).
Conflict of interest statement
MGM participated as a faculty member and advisor in scientific meetings and courses under the sponsorship of ABC Farmaceutici, Almirall, AstraZeneca, Chiesi Farmaceutici, GlaxoSmithKline and Novartis, was a consultant to Chiesi Farmaceutici and GSK, and her department was funded by GSK and Novartis. CC received honoraria for lectures from AstraZeneca, GSK, Sanofi and Novartis, and support for attending meetings and/or travel received from AstraZeneca, GSK, Sanofi and Novartis. LC has participated as advisor in scientific meetings under the sponsorship of Boehringer Ingelheim and Novartis, received nonfinancial support from AstraZeneca, received a research grant partially funded by Chiesi Farmaceutici, Boehringer Ingelheim, Novartis, and Almirall; has been a consultant to ABC Farmaceutici, Edmond Pharma, Zambon, Verona Pharma, and Ockham Biotech; his department was funded by Almirall, Boehringer Ingelheim, Chiesi Farmaceutici, Novartis, and Zambon. MC participated as a faculty member and advisor in scientific meetings and courses under the sponsorship of Abdi Ibrahim, Alkem, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Cipla, Eurodrug, GSK, Glenmark, Lallemand, Mankind Pharma, Menarini Group, Mundipharma, Novartis, Pfizer, Recipharm, Sanofi, Teva, Verona Pharma and Zambon, and is or was a consultant to ABC Farmaceutici, AstraZeneca, Chiesi Farmaceutici, GSK, Lallemand, Novartis, Ockham Biotech, Recipharm, Verona Pharma and Zambon. CP has acted as a consultant to Eurodrug, Recipharm, Glycosynnovation and PrEP Biopharma, and also holds equity in Verona Pharma. BR and CB declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

