RUNX1 promotes angiogenesis in colorectal cancer by regulating the crosstalk between tumor cells and tumor associated macrophages
- PMID: 38419056
- PMCID: PMC10903076
- DOI: 10.1186/s40364-024-00573-1
RUNX1 promotes angiogenesis in colorectal cancer by regulating the crosstalk between tumor cells and tumor associated macrophages
Abstract
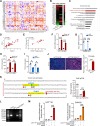
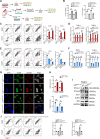
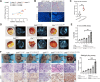
Colorectal cancer (CRC) is a common malignancy worldwide. Angiogenesis and metastasis are the critical hallmarks of malignant tumor. Runt-related transcription factor 1 (RUNX1), an efficient transcription factor, facilitates CRC proliferation, metastasis and chemotherapy resistance. We aimed to investigate the RUNX1 mediated crosstalk between tumor cells and M2 polarized tumor associated macrophages (TAMs) in CRC, as well as its relationship with neoplastic angiogenesis. We found that RUNX1 recruited macrophages and induced M2 polarized TAMs in CRC by promoting the production of chemokine 2 (CCL2) and the activation of Hedgehog pathway. In addition, we found that the M2 macrophage-specific generated cytokine, platelet-derived growth factor (PDGF)-BB, promoted vessel formation both in vitro and vivo. PDGF-BB was also found to enhance the expression of RUNX1 in CRC cell lines, and promote its migration and invasion in vitro. A positive feedback loop of RUNX1 and PDGF-BB was thus formed. In conclusion, our data suggest that RUNX1 promotes CRC angiogenesis by regulating M2 macrophages during the complex crosstalk between tumor cells and TAMs. This observation provides a potential combined therapy strategy targeting RUNX1 and TAMs-related PDGF-BB in CRC.
Keywords: Angiogenesis; Colorectal cancer; M2 polarization; RUNX1; Tumor associated macrophages.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
LinkOut - more resources
Full Text Sources

